- Clinical Technology
- Adult Immunization
- Hepatology
- Pediatric Immunization
- Screening
- Psychiatry
- Allergy
- Women's Health
- Cardiology
- Pediatrics
- Dermatology
- Endocrinology
- Pain Management
- Gastroenterology
- Infectious Disease
- Obesity Medicine
- Rheumatology
- Nephrology
- Neurology
- Pulmonology
Treating sepsis: An update on the latest therapies, part 1
A milestone has been reached in the treatment of sepsis—the institution of protocolized management that starts in the emergency department. Early goal-directed therapy, with targeted fluid resuscitation and measures of oxygen delivery, has been shown to improve survival in patients with septic shock. Although initiating aggressive fluid resuscitation is the first priority, it is also essential to rapidly obtain cultures and infuse broad-spectrum antibiotics. Norepinephrine is a more potent vasoconstrictor than dopamine and may be more effective in treating hypotension in patients with septic shock. Vasopressin is an effective second-line agent. Treatment with recombinant human activated protein C at 24 µg/kg/h for 96 hours has been shown to reduce mortality in patients with sepsis; its benefit is greatest in the most acutely ill patients. (J Respir Dis. 2009;30(1-2))
ABSTRACT: A milestone has been reached in the treatment of sepsis-the institution of protocolized management that starts in the emergency department. Early goal-directed therapy, with targeted fluid resuscitation and measures of oxygen delivery, has been shown to improve survival in patients with septic shock. Although initiating aggressive fluid resuscitation is the first priority, it is also essential to rapidly obtain cultures and infuse broad-spectrum antibiotics. Norepinephrine is a more potent vasoconstrictor than dopamine and may be more effective in treating hypotension in patients with septic shock. Vasopressin is an effective second-line agent. Treatment with recombinant human activated protein C at 24 µg/kg/h for 96 hours has been shown to reduce mortality in patients with sepsis; its benefit is greatest in the most acutely ill patients. (J Respir Dis. 2009;30[1-2])
For the diagnosis and management of sepsis, the past 10 years have brought significant changes. Initial treatment involving fluid resuscitation, antibiotics, and source control is similar to what it was a decade ago, but we now realize that the speed of diagnosis coupled with expedited management is critical.1 New ways to support failing organ systems2 and the introduction of a pharmacological agent to reduce mortality3 have shed light on this disease and have given clinicians new optimism. Slowly but surely, we are making the transition toward delivering a multitude of treatments simultaneously in an effort to improve clinical care.
In this 2-part article, we will review the latest strategies for managing sepsis.
THE SEPSIS CONTINUUM
Sepsis is a significant public health problem that occurs at the rate of approximately 3 cases per 1000 persons, affecting nearly 900,000 persons every year in the United States and nearly 20,000,000 worldwide.4 As the leading cause of death in noncoronary ICUs and the 10th leading cause of death overall in the United States,4-6 sepsis has a tremendous economic and social burden. Not only does sepsis mortality range from 20% to 80% depending on illness severity4,5,7-9 but survivors experience a significant increase in morbidity and reduced quality of life.10,11
Recent data from the United States show that the incidence of sepsis is increasing, with a growing number of deaths despite an overall decrease in proportionate mortality.4 With the aging population, increased use of immunosuppressive drugs, emergence of HIV, increasing microbial resistance, and expanding availability of health care and health care interventions, the incidence of sepsis will continue to increase globally.
DEFINITION AND CASE FINDING
The diagnosis of sepsis can still be perplexing despite a well-accepted consensus definition. In 1992, the American College of Chest Physicians/Society of Critical Care Medicine consensus conference arrived at the current definition of sepsis as a systemic inflammatory syndrome (defined by a change in 2 or more abnormal clinical findings: temperature, heart rate, respiration rate, and white blood cell count) with a concomitant pathological infection (Table 1).12
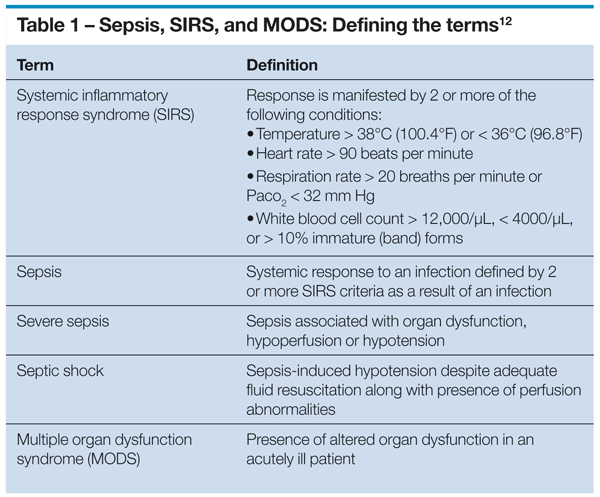
Sepsis severity was defined by the addition of acute organ dysfunction, hypoperfusion, or hypotension, based on criteria proposed by Marshall and associates13 or by criteria used for Sequential Organ Failure Assessment score.14 Septic shock refers to sepsis-induced hypotension that persists despite adequate fluid resuscitation.
Sepsis is difficult to diagnose, particularly in the earlier stages when the symptoms may be subtle. Although it would be clearly beneficial to have an accurate test that identifies sepsis, no diagnostic test exists and the recognition of early sepsis often requires an astute clinician who is knowledgeable about the sepsis syndrome.
Various biomarkers have been evaluated for diagnosis, risk stratification, and prognosis in sepsis, including procalcitonin, C-reactive protein (CRP), B-type natriuretic peptide,15,16 and protein C. Although the procalcitonin level has limited diagnostic value in patients with systemic inflammatory response syndrome from other causes,17 it appears to be a better marker for illness severity and prognosis than the CRP level.18-21 The directional change in protein C levels have been shown to correlate with outcomes in patients with severe sepsis22 and may prove to be useful tools in the future.
A soluble triggering receptor expressed on myeloid cells-1, a recently discovered receptor expressed on the surface of neutrophils, has been reported to trigger the synthesis of proinflammatory cytokines in presence of microbial products23 and has been found to predict outcome in patients with sepsis.24 However, none of the above-mentioned biomarkers has thus far proved clinically useful for the diagnosis of sepsis.
PATHOPHYSIOLOGY
Over the years, a considerable amount has changed in our thinking about sepsis pathophysiology. Initially considered a syndrome of exaggerated inflammation,12 sepsis is now recognized as a complex set of interactions between the inciting microbe, the host immune response, and the inflammatory and coagulation pathways.
Inflammation and immune response
An infectious insult, classically described as bacterial endotoxin, initiates a pathophysiological cascade involving pattern-recognition receptors called toll-like receptors.25 Binding of these receptors to microorganisms results in the release of a number of proinflammatory cytokines, especially tumor necrosis factor-α, which are important for host immune defense and resolution of the inflammatory response as they interact with invading pathogens.26,27 These pathways often yield further activation of other myeloid-derived and/or endothelial cells.28
Simultaneously, activation of anti-inflammatory pathways may lessen the inflammatory response.29
Coagulation imbalance
In recent years, it has been recognized that the coagulation system acts in concert with the inflammatory cascade in the pathophysiology of sepsis.30-33 These abnormalities range from subclinical prolongation of clotting times to fulminant disseminated intravascular coagulation, characterized by global microvascular thrombosis and bleeding.30 Amelioration of this coagulopathy appears to attenuate organ failure and, subsequently, survival.3,32
The protein C pathway serves as an anticoagulant system, promoting fibrinolysis by inhibiting thrombosis and inflammation.34 Thrombin binds to thrombomodulin at the endothelial protein C receptor (EPCR) on the endothelium, resulting in a complex that rapidly activates protein C, which binds to protein S, inactivating factors Va and VIIIa.35,36 EPCR deletion exaggerates the host responses to lipopolysaccharide,33 suggesting that EPCR is important in controlling endotoxin-induced coagulation and inflammatory responses.
Multiple organ dysfunction syndrome
Multiorgan system dysfunction in sepsis may be partially caused by relative host immunosuppression. Stimulation by an infectious insult may cause a shift to an anti-inflammatory milieu, resulting in production of anti-inflammatory cytokines.37,38
In addition, apoptosis of circulating immune, epithelial, and endothelial cells induced by endotoxin and proinflammatory cytokines inducing cytopathic hypoxia can contribute to this immunosuppression.39,40 These altered signaling pathways lead to tissue injury and multiorgan dysfunction.
MANAGEMENT
In the past decade, there have been significant developments demonstrating the importance of speed and accuracy in diagnosing sepsis and instituting appropriate care.
Initial resuscitation
• Early goal-directed therapy: A milestone has been reached in the treatment of sepsis-the institution of protocolized management that starts in the emergency department (ED). Early goal-directed therapy, with targeted fluid resuscitation and measures of oxygen delivery, has been shown to improve survival in patients with septic shock.1
This evidence comes from a randomized controlled single-center trial that assigned 263 patients with severe sepsis or septic shock to receive protocolized early goal-directed therapy during the first 6 hours in the ED or to receive standard therapy before ICU admission.1 Depending on whether patients had central venous catheters or pulmonary artery catheters, central venous oxygen saturation (ScvO2) or mixed venous oxygen saturation (SvO2) was continuously measured in those receiving early goal-directed therapy and dictated further therapy if goals were not achieved.
Resuscitation goals for the initial 6 hours included a central venous pressure of 8 to 12 mm Hg, mean arterial pressure (MAP) greater than 65 mm Hg, urinary output greater than 0.5 mL/kg/h, and an ScvO2 or SvO2 of greater than 70% or 65%, respectively. If ScvO2 was less than 70%, packed red blood cells (PRBCs) were transfused to achieve a hematocrit level of 30%. If central venous pressure, MAP, and hematocrit were optimized and ScvO2 remained less than 70%, dobutamine was added to increase cardiac output and oxygen delivery (Table 2).
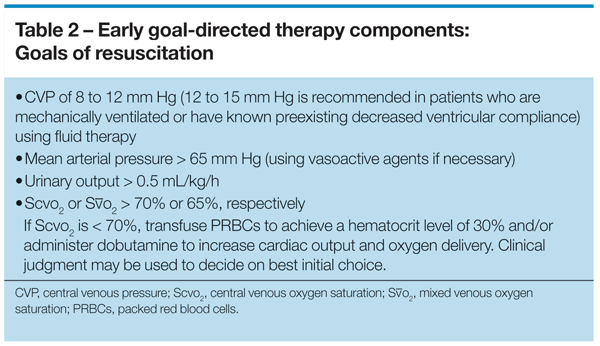
Resuscitation to these goals within 6 hours reduced in-hospital mortality in patients with severe sepsis from 46.5% to 30.5% (P < .009) and reduced mortality at 28 days (P = .01) and at 60 days (P = .03).1 Although this evidence comes from a single-center trial, this study reliably showed that early goal-directed resuscitation improved survival for ED patients with septic shock, and these goals have now become part of the treatment protocol in patients with sepsis-induced shock in recent Surviving Sepsis Campaign (SSC) guidelines.41 In addition, a growing number of studies have supported the use of early protocolized resuscitation to improve outcomes.42-45
During the initial 6 hours, patients in the early goal-directed therapy group received significantly more fluids, PRBCs, and inotropic support than those in the standard therapy group, and they required less intensive hospital care and had less severe illness through 72 hours.1 Of note, in mechanically ventilated patients, a higher target central venous pressure of 12 to 15 mm Hg is generally recommended to account for the impediment to cardiac filling caused by higher intrathoracic pressures.46
The design of this trial does not allow one to assess the relative contributions of the various components, particularly PRBC transfusion and dobutamine. Thus, once a patient has been adequately resuscitated with fluid therapy, it is up to the clinician whether PRBC transfusion or dobutamine is the best initial choice to maximize oxygen delivery.41
• Colloids versus crystalloids: Meta-analyses of studies evaluating the use of crystalloids versus colloids for resuscitation have had conflicting results, such that recent SSC guidelines cannot recommend with good evidence one type of fluid over the other.47-50 Although some studies have indicated that certain colloids cause organ damage, the Saline Versus Albumin Fluid Evaluation (SAFE) study, a multicenter randomized trial that compared 4% albumin with 0.9% normal saline in patients requiring fluid resuscitation, indicated that albumin was as safe as crystalloid and equally effective.51
There was no difference in 28-day all-cause mortality rate, organ dysfunction, the duration of mechanical ventilation, or ICU or hospital length of stay.51 There was a nonsignificant decrease in mortality in the subgroup of patients with severe sepsis treated with albumin (P = .09) and an increase in mortality in albumin-treated patients who had traumatic brain injury.51,52
A few studies have evaluated the use of hydroxyethyl starch (HES) for fluid resuscitation.53,54 In animal models, HES has been shown to improve microcirculation during endotoxemia,55-58 but it also has been known to have adverse effects, such as coagulopathy and renal failure.59,60 In a multicenter randomized trial, Schortgen and coworkers54 found that use of HES was an independent risk factor for acute renal failure in patients with severe sepsis or septic shock.
A recent randomized controlled trial comparing the use of HES with that of Ringer lactate in patients with severe sepsis and septic shock was suspended after the first preplanned interim analysis because of an increased risk of renal failure and a trend toward increased mortality at 90 days in the HES group.61
Antibiotic therapy
Although initiating aggressive fluid resuscitation is the first priority when managing severe sepsis or septic shock, it is also important to rapidly obtain cultures of suspected body fluids or blood and promptly infuse broad-spectrum antibiotics. It is advisable to obtain cultures before starting antimicrobial therapy (since rapid sterilization of blood cultures can occur within a few hours), but antibiotic therapy should not be unduly delayed.
A large study of 18,209 Medicare patients hospitalized with community-acquired pneumonia showed that antibiotic use within 4 hours of arrival at the hospital was associated with decreased mortality and hospital length of stay.62 In addition, in the presence of septic shock, each hour delay in antimicrobial administration has been found to decrease survival.63
Initially, empirical antibiotic therapy should be broad enough to cover all possible pathogens. It is important for physicians to be aware of their hospital’s antibiotic profile, in addition to the virulence patterns of pathogens in the community. When selecting antibiotics, care providers must not only guess early, but they must also guess right. Failure to initiate adequate antimicrobial therapy correlates with increased morbidity and mortality in patients with sepsis admitted to the ICU.64-67
Once the causative pathogen has been identified, the antibiotic regimen should be narrowed. However, this is not an appropriate initial strategy, and the desire to minimize superinfections and resistance should not take precedence over adequately treating patients with severe sepsis and septic shock.
Although combination therapy has never been shown to significantly improve outcomes,68-70 multiple antibiotics may be useful in certain situations. Recent guidelines suggest that combination therapy be used for neutropenic patients and for patients with known or suspected Pseudomonas infections as a cause of severe sepsis.41 When used empirically, however, combination therapy should not be continued for longer than 3 to 5 days.
An observational study of patients with ventilator-associated pneumonia (VAP) showed that monotherapy was associated with inappropriate therapy, which, in turn, was associated with increased in-hospital mortality.71 This suggests that initial use of combination therapy reduces the likelihood of inappropriate therapy, thereby reducing the risk of death. Most recently, a randomized multicenter clinical trial showed that although there was no difference in 28-day mortality between patients with VAP who were treated with combination antibiotic therapy and those who were treated with monotherapy, the subgroup of patients at high risk for difficult-to-treat gram-negative bacterial infection had better microbiological and clinical outcomes with combination antibiotic therapy.68 The recommended duration of antimicrobial therapy is 7 to 10 days, although longer courses may be appropriate in some patients.41
Hemodynamic management-use of vasopressors
Despite fluid resuscitation, vasopressor therapy is occasionally required. Below a certain MAP, autoregulation of pressure in vascular beds can be lost and perfusion becomes linearly dependent on pressure.72,73 However, loss of auto-regulation can occur at different levels in different organs.
Titration of norepinephrine to an MAP as low as 65 mm Hg has been shown to preserve tissue perfusion in a small study of 10 patients,72 and increasing the vasopressor dose to maintain a higher MAP (85 mm Hg) does not significantly affect metabolic parameters or renal function.72,74
However, the patient’s baseline blood pressure should also be considered. Someone with chronic systemic arterial hypertension might require an MAP greater than 65 mm Hg to maintain tissue perfusion, while a lower MAP may be adequate in a patient with chronic hepatic failure. Therefore, health care providers must always supplement arterial pressure with other measures of global tissue perfusion, such as ScvO2, tissue oximetry, blood lactate levels, delayed capillary refill, and urinary output.41,72
There has been a long-standing debate about vasopressor superiority. Although these discussions are intellectually stimulating, remember that each catecholamine agent has variable receptor-mediated effects, so distinct clinical situations may require different vasopressors. For example, norepinephrine has potent α-adrenergic effects and less potent β-adrenergic effects, while dopamine’s receptor effects are dose-dependent.
Some studies suggest that norepinephrine or dopamine may have some advantages over other vasopressors, and recent guidelines recommend either norepinephrine or dopamine as a first-choice vasopressor agent to correct hypotension in septic shock.41 Norepinephrine is a more potent vasoconstrictor than dopamine and may be more effective in treating hypotension in patients with septic shock. In the only randomized trial comparing these vasopressors, the ability of dopamine and norepinephrine to reverse hemodynamic derangements associated with septic shock was evaluated in 32 patients.75 More patients were successfully treated with norepinephrine, including those who did not respond to dopamine.
In a larger, observational study, the use of norepinephrine as the vasopressor of choice was associated with lower hospital mortality in patients with septic shock.76 A prospective, double-blind, multicenter, randomized, controlled trial with 1600 patients is under way to compare the efficacy of dopamine with that of norepinephrine in the treatment of shock.77
Epinephrine has been suggested as the first-choice alternative in septic shock that is poorly responsive to norepinephrine or dopamine. Annane and colleagues78 conducted a randomized controlled trial of 330 patients with septic shock to compare the efficacy of norepinephrine plus dobutamine with that of epinephrine. They found that there was no difference in efficacy or safety between the 2 groups. In the past, smaller studies have found that patients treated with epinephrine tend to have inadequate, albeit transient, splanchnic oxygen utilization, resulting in a higher incidence of gastric mucosal acidosis.79
Vasopressin, an endogenous hormone synthesized in the hypothalamus, has emerged as an adjunct to catecholamines for patients with septic shock. Vasopressin levels have been found to be lower than expected in patients with septic shock, suggesting a relative vasopressin deficiency state.80,81 In addition, vasopressin has been found to spare catecholamine use and have other beneficial physiological effects.80-84
Recently, a large, multicenter, randomized, double-blind trial of approximately 800 patients was conducted to determine whether norepinephrine and vasopressin at 0.03 U/min decreased mortality compared with norepinephrine alone.85 There was no difference in mortality, ICU and hospital length of stay, days alive and free of vasopressor use, use of corticosteroids, or organ dysfunction, but the dose of norepinephrine infusion was significantly lower in the group receiving vasopressin. Although there was no difference in the rates of adverse events overall, there was a trend toward a higher rate of cardiac arrest in the norepinephrine group and a trend toward a higher rate of digital ischemia in the vasopressin plus norepinephrine group.
This study demonstrated that although vasopressin is an effective second-line agent, it is not more effective than using norepinephrine alone. Vasopressin, however, may be used at low doses (0.03 U/min), particularly for refractory hypotension, and should be reserved for a specific subset of patients (those without coronary or mesenteric ischemia or those who are unable to tolerate high doses of norepinephrine).
Recombinant human activated protein C
The PROtein C Worldwide Evaluation in Severe Sepsis (PROWESS) study randomized 1690 patients with severe sepsis to receive either recombinant human activated protein C (rhAPC) at 24 µg/kg/h for 96 hours or placebo.86 Treatment reduced absolute mortality by 6.1% and relative mortality by 19.4% (P = .005). The benefit was greatest-a decrease in absolute mortality of 13%-in the most acutely ill patients (those with APACHE II scores of 25 or higher).86
Subsequently, in the Administration of Drotrecogin Alfa [Activated] in Early Stage Severe Sepsis (ADDRESS) trial, 2613 patients assessed to have a low risk of death (generally, an APACHE II score of less than 25 or single-organ dysfunction) were randomized to receive rhAPC or placebo; this study found no difference in 28-day mortality.87 Importantly, the ADDRESS trial showed that the 28-day mortality rate was significantly higher in patients who had had recent surgery and who had single-organ dysfunction who were treated with rhAPC (20.7% vs 14.1%, P = .03).
Faced with critically ill patients with severe sepsis, intensivists must decide whom to treat with rhAPC based on the extensive list of exclusion criteria in the clinical studies and contraindications. Serious adverse events did not differ in the 2 studies except for serious bleeding during infusion, which was increased in the group that received rhAPC (PROWESS, 3.5% vs 2%, P = .06; and ADDRESS, 3.9% vs 2.2%, P < .01)3,87 and tended to occur in patients with a predisposition to bleeding (such as those with GI ulceration or coagulopathy and those undergoing procedures).
Additional safety information came from an open-label observational study, Extended Evaluation of Recombinant Human Activated Protein C (ENHANCE), which showed a 28-day all-cause mortality similar to that of PROWESS (25.3% vs 24.7%) but a 3.6% increase in serious bleeding during infusion and a 6.5% increase at 28 days.88 In addition, the rate of intracranial hemorrhage was increased in patients who received rhAPC in ENHANCE versus PROWESS (1.5% vs 0.2%).88
Recently, the use of prophylactic heparin was evaluated in patients who received rhAPC.89 There was no harmful interaction between rhAPC and heparin and, in fact, there was a nonsignificant reduction in mortality in patients who received heparin.89
Given all this information, critical care physicians must decide which patients will benefit from rhAPC and whether the benefit exceeds the risk in each patient. rhAPC is an expensive drug, but multiple analyses have reported its cost-effectiveness in patients who have severe sepsis and a high predicted mortality at baseline.90-92 Although the conclusions from PROWESS and ADDRESS are limited, there still is a probable mortality reduction in patients with sepsis-induced organ dysfunction associated with a high risk of death based on clinical assessment, most of whom will have an APACHE II score greater than 25 or multiorgan failure. Therefore, it is suggested that rhAPC be considered for these patients, presuming there are no contraindications.41
Corticosteroids
The past decade has seen a considerable debate and the emergence of new evidence regarding the use of corticosteroids in septic shock. In the past, randomized clinical trials and meta-analyses have shown that high-dose corticosteroid therapy is ineffective in patients with severe sepsis or septic shock.93-96 Until recently, there was 1 multicenter randomized controlled trial that suggested better shock reversal and a survival benefit in patients with vasopressor-unresponsive septic shock and relative adrenal insufficiency, defined as a post–adrenocorticotropic hormone (ACTH) cortisol level increase of 9 µg/dL or less.97 Two smaller single-center studies also suggested that there was a greater incidence of shock reversal with corticosteroids.98-99
A large European multicenter trial, Corticosteroid Therapy of Septic Shock (CORTICUS), randomized 499 patients with septic shock to receive either low-dose hydrocortisone therapy or placebo for 5 days.100 The authors concluded that at 28 days, there was no significant difference in mortality between patients in the 2 treatment groups, irrespective of any response to ACTH.100 While corticosteroids hastened the reversal of septic shock, they were also associated with a greater risk of nosocomial infections and recurrent sepsis.100 These results suggest that ACTH stimulation testing is not useful in predicting which patients with sepsis may benefit from corticosteroids and that corticosteroid therapy in general does not improve clinical outcomes in patients with septic shock.
Corticosteroids are not without adverse effects. These drugs are immunosuppressive, potentially leading to secondary infections and impaired wound healing, and can cause myopathy, hyperglycemia, and hypernatremia.98-100 Thus, corticosteroid therapy should be discontinued as early as possible.41 However, to date no study has compared a fixed duration of corticosteroid therapy followed by tapering over several days92,93 or abrupt discontinuation91 versus tapering therapy after shock resolution,99 so it remains uncertain whether outcome is affected by tapering of corticosteroids.
However, despite these controversies, corticosteroids are still suggested only for patients with septic shock, since no studies suggest a benefit in patients with less severe forms of sepsis whose blood pressure is poorly responsive to fluids and vasopressor therapy.41
Treatment of anemia in sepsis
Anemia is a common feature among critically ill patients with sepsis who often require transfusions of PRBCs.101 Hemoglobin concentrations typically decline during the first few days of an ICU stay, but while they tend to stabilize in patients without sepsis, hemoglobin concentrations continue to decline in patients with sepsis.102 To date, the optimum hemoglobin level in patients with sepsis has not been evaluated, but it certainly varies according to the phase (early vs late) of sepsis.
Two main randomized controlled trials have evaluated transfusion strategies in patients with sepsis.1,103 Early goal-directed therapy includes transfusion of PRBCs to a target hematocrit of 30% or higher during the first 6 hours of resuscitation for septic shock.1 Although not limited to patients with sepsis, the Transfusion Requirements in Critical Care (TRICC) trial compared 2 transfusion strategies in 838 euvolemic critically ill patients.103 After initial resuscitation, the patients were randomized to either a restrictive (maintenance of hemoglobin level at 7.0 to 9.0 g/dL with a threshold of 7.0 g/dL) or a liberal (maintenance of hemoglobin level of 10.0 to 12.0 g/dL with a threshold of 10.0 g/dL) transfusion strategy that was adhered to throughout the patient’s ICU stay.
There was no difference in 30-day all-cause mortality between the 2 groups overall, but there was improved survival in the younger and less severely ill patients.103 Sepsis was the primary diagnosis in only 5% of patients, limiting the interpretation of these results for this population. Together, these studies suggest that PRBC transfusion is valuable during the early stage of sepsis as opposed to later stages when patients are more likely to be euvolemic-transfusion to a hematocrit level of 30% or higher decreases mortality during the first 6 hours of resuscitation, while maintenance of hemoglobin levels of 7.0 to 9.0 g/dL is adequate after initial resuscitation.
Recombinant human erythropoietin has also been evaluated as a therapy for critically ill anemic patients. Patients with sepsis exhibit inappropriately low levels of erythropoietin,104,105 and 2 previous trials involving critically ill patients showed that erythropoietin therapy reduced red cell transfusions but did not decrease mortality.106,107
In a large randomized trial of 1460 patients, of which 13% had sepsis, erythropoietin therapy did not reduce the incidence of PRBC transfusion among critically ill patients.108 While mortality was reduced in the trauma patients, the incidence of thrombotic events was increased in all patients who received erythropoietin therapy.108 As such, current guidelines do not recommend erythropoietin for the treatment of anemia associated with sepsis.41
[Editor’s note: In part 2, Drs Cribbs and Martin will continue their review of the management of sepsis.]
References:
REFERENCES
1. Rivers E, Nguyen B, Havstad S, et al; Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368-1377.
2. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301-1308.
3. Bernard GR, Vincent JL, Laterre PF, et al; Recombinant human protein C Worldwide Evaluation in Severe Sepsis (PROWESS) Study Group. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699-709.
4. Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546-1554.
5. Alberti C, Brun-Buisson C, Burchardi H, et al. Epidemiology of sepsis and infection in ICU patients from an international multicentre cohort study [published correction appears in Intensive Care Med. 2002;28:525-526]. Intensive Care Med. 2002;28:108-121.
6. Parrillo JE, Parker MM, Natanson C, et al. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med. 1990;113:227-242.
7. Brun-Buisson C, Doyon F, Carlet J, et al. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults. A multicenter prospective study in intensive care units. French ICU Group for Severe Sepsis. JAMA. 1995;274:968-974.
8. Padkin A, Goldfrad C, Brady AR, et al. Epidemiology of severe sepsis occurring in the first 24 hrs in intensive care units in England, Wales, and Northern Ireland. Crit Care Med. 2003;31:2332-2338.
9. Brun-Buisson C, Meshaka P, Pinton P, Vallet B; EPISEPSIS Study Group. EPISEPSIS: a reappraisal of the epidemiology and outcome of severe sepsis in French intensive care units. Intensive Care Med. 2004;30:580-588.
10. Heyland DK, Hopman W, Coo H, et al. Long-term health-related quality of life in survivors of sepsis. Short Form 36: a valid and reliable measure of health-related quality of life. Crit Care Med. 2000;28:3599-3605.
11. Perl TM, Dvorak L, Hwang T, Wenzel RP. Long-term survival and function after suspected gram-negative sepsis. JAMA. 1995;274:338-345.
12. Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644-1655.
13. Marshall JC, Cook DJ, Christou NV, et al. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23:1638-1652.
14. Ferreira FL, Bota DP, Bross A, et al. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286:1754-1758.
15. Kandil E, Burack J, Sawas A, et al. B-type natriuretic peptide: a biomarker for the diagnosis and risk stratification of patients with septic shock. Arch Surg. 2008;143:242-246.
16. Meyer B, Huelsmann M, Wexberg P, et al. N-terminal pro-B-type natriuretic peptide is an independent predictor of outcome in an unselected cohort of critically ill patients. Crit Care Med. 2007;35:2268-2273.
17. Giamarellos-Bourboulis EJ, Giannopoulou P, Grecka P, et al. Should procalcitonin be introduced in the diagnostic criteria for the systemic inflammatory response syndrome and sepsis? J Crit Care. 2004;19:152-157.`
18. Uzzan B, Cohen R, Nicolas P, et al. Procalcitonin as a diagnostic test for sepsis in critically ill adults and after surgery or trauma: a systematic review and meta-analysis. Crit Care Med. 2006;34:1996-2003.
19. Claeys R, Vinken S, Spapen H, et al. Plasma procalcitonin and C-reactive protein in acute septic shock: clinical and biological correlates. Crit Care Med. 2002;30:757-762.
20. Meisner M, Tschaikowsky K, Palmaers T, Schmidt J. Comparison of procalcitonin (PCT) and C-reactive protein (CRP) plasma concentrations at different SOFA scores during the course of sepsis and MODS. Crit Care. 1999;3:45-50.
21. Harbarth S, Holeckova K, Froidevaux C, et al; Geneva Sepsis Network. Diagnostic value of procalcitonin, interleukin-6, and interleukin-8 in critically ill patients admitted with suspected sepsis. Am J Respir Crit Care Med. 2001;164:396-402.
22. Shorr AF, Bernard GR, Dhainaut JF, et al. Protein C concentrations in severe sepsis: an early directional change in plasma levels predicts outcome. Crit Care. 2006;10:R92.
23. Bouchon A, Dietrich J, Colonna M. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol. 2000;164:4991-4995.
24. Gibot S, Cravoisy A, Kolopp-Sarda MN, et al. Time-course of sTREM (soluble triggering receptor expressed on myeloid cells)-1, procalcitonin, and C-reactive protein plasma concentrations during sepsis. Crit Care Med. 2005;33:792-796.
25. Modlin RL, Brightbill HD, Godowski PJ. The toll of innate immunity on microbial pathogens. N Engl J Med. 1999;340:1834-1835.
26. Fujita M, Kuwano K, Kunitake R, et al. Endothelial cell apoptosis in lipopolysaccharide-induced lung injury in mice. Int Arch Allergy Immunol. 1998;117:202-208.
27. Yamada M, Kubo H, Kobayashi S, et al. Bone marrow-derived progenitor cells are important for lung repair after lipopolysaccharide-induced lung injury [published correction appears in J Immunol. 2004;173:4755]. J Immunol. 2004;172:1266-1272.
28. Aird WC. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood. 2003;101:3765-3777.
29. Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000;117:1162-1172.
30. Levi M, Keller TT, van Gorp E, ten Cate H. Infection and inflammation and the coagulation system. Cardiovasc Res. 2003;60:26-39.
31. Levi M, de Jonge E, van der Poll T. Sepsis and disseminated intravascular coagulation. J Thromb Thrombolysis. 2003;16:43-47.
32. Miller DL, Welty-Wolf K, Carraway MS, et al. Extrinsic coagulation blockade attenuates lung injury and proinflammatory cytokine release after intratracheal lipopolysaccharide. Am J Respir Cell Mol Biol. 2002;26:650-658.
33. Zheng X, Li W, Song Y, et al. Non-hematopoietic EPCR regulates the coagulation and inflammatory responses during endotoxemia. J Thromb Haemost. 2007;5:1394-1400.
34. Esmon CT. The protein C anticoagulant pathway. Arterioscler Thromb. 1992;12:135-145.
35. Esmon CT. Regulation of blood coagulation. Biochim Biophys Acta. 2000;1477:349-360.
36. Stearns-Kurosawa DJ, Kurosawa S, Mollica JS, et al. The endothelial cell protein C receptor augments protein C activation by the thrombin-thrombomodulin complex. Proc Natl Acad Sci U S A. 1996;93:10212-10216.
37. Ertel W, Kremer JP, Kenney J, et al. Downregulation of proinflammatory cytokine release in whole blood from septic patients. Blood. 1995;85:1341-1347.
38. Gogos CA, Drosou E, Bassaris HP, Skoutelis A. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J Infect Dis. 2000;181:176-180.
39. Chang KC, Unsinger J, Davis CG, et al. Multiple triggers of cell death in sepsis: death receptor and mitochondrial-mediated apoptosis. FASEB J. 2007;21:708-719.
40. Hotchkiss RS, Tinsley KW, Swanson PE, et al. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol. 2001;166:6952-6963.
41. Dellinger RP, Levy MM, Carlet JM, et al; International Surviving Sepsis Campaign Guidelines Committee, American Association of Critical-Care Nurses, American College of Chest Physicians, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008 [published correction appears in Crit Care Med. 2008;36:1394-1396]. Crit Care Med. 2008;36:296-327.
42. Micek ST, Roubinian N, Heuring T, et al. Before-after study of a standardized hospital order set for the management of septic shock. Crit Care Med. 2006;34:2707-2713.
43. Nguyen HB, Corbett SW, Steele R, et al. Implementation of a bundle of quality indicators for the early management of severe sepsis and septic shock is associated with decreased mortality. Crit Care Med. 2007;35:1105-1112.
44. Sebat F, Johnson D, Musthafa AA, et al. A multidisciplinary community hospital program for early and rapid resuscitation of shock in nontrauma patients. Chest. 2005;127:1729-1743.
45. Talmor D, Greenberg D, Howell MD, et al. The costs and cost-effectiveness of an integrated sepsis treatment protocol. Crit Care Med. 2008;36:1168-1174.
46. Bendjelid K, Romand JA. Fluid responsiveness in mechanically ventilated patients: a review of indices used in intensive care. Intensive Care Med. 2003;29:352-360.
47. Choi PT, Yip G, Quinonez LG, Cook DJ. Crystalloids vs colloids in fluid resuscitation: a systematic review. Crit Care Med. 1999;27:200-210.
48. Schierhout G, Roberts I. Fluid resuscitation with colloid or crystalloid solutions in critically ill patients: a systematic review of randomised trials. BMJ. 1998;316:961-964.
49. Wilkes MM, Navickis RJ. Patient survival after human albumin administration. A meta-analysis of randomized, controlled trials. Ann Intern Med. 2001;135:149-164.
50. Vincent JL, Navickis RJ, Wilkes MM. Morbidity in hospitalized patients receiving human albumin: a meta-analysis of randomized, controlled trials. Crit Care Med. 2004;32:2029-2038.
51. Finfer S, Bellomo R, Boyce N, et al; SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247-2256.
52. Myburgh J, Cooper DJ, Finfer S, et al. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med. 2007;357:874-884.
53. Sakr Y, Dubois MJ, De Backer D, et al. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med. 2004;32:1825-1831.
54. Schortgen F, Lacherade JC, Bruneel F, et al. Effects of hydroxyethylstarch and gelatin on renal function in severe sepsis: a multicentre randomised study. Lancet. 2001;357:911-916.
55. Hoffmann JN, Vollmar B, Laschke MW, et al. Hydroxyethyl starch (130 kD), but not crystalloid volume support, improves microcirculation during normotensive endotoxemia. Anesthesiology. 2002;97:460-470.
56. Kellum JA. Fluid resuscitation and hyperchloremic acidosis in experimental sepsis: improved short-term survival and acid-base balance with Hextend compared with saline. Crit Care Med. 2002;30:300-305.
57. Kupper S, Mees ST, Gassmann P, et al. Hydroxy-ethyl starch normalizes platelet and leukocyte adhesion within pulmonary microcirculation during LPS-induced endotoxemia. Shock. 2007;28:300-308.
58. Maciel F, Mook M, Zhang H, Vincent JL. Comparison of hypertonic with isotonic saline hydroxyethyl starch solution on oxygen extraction capabilities during endotoxic shock. Shock. 1998;9:33-39.
59. Dickenmann M, Oettl T, Mihatsch MJ. Osmotic nephrosis: acute kidney injury with accumulation of proximal tubular lysosomes due to administration of exogenous solutes. Am J Kidney Dis. 2008;51:491-503.
60. Nielsen VG. Hydroxyethyl starch enhances fibrinolysis in human plasma by diminishing alpha2-antiplasmin-plasmin interactions. Blood Coagul Fibrinolysis. 2007;18:647-656.
61. Brunkhorst FM, Engel C, Bloos F, et al; German Competence Network Sepsis (SepNet). Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125-139.
62. Houck PM, Bratzler DW, Nsa W, et al. Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community-acquired pneumonia. Arch Intern Med. 2004;164:637-644.
63. Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589-1596.
64. Garnacho-Montero J, Garcia-Garmendia JL, Barrero-Almodovar A, et al. Impact of adequate empirical antibiotic therapy on the outcome of patients admitted to the intensive care unit with sepsis. Crit Care Med. 2003;31:2742-2751.
65. Harbarth S, Garbino J, Pugin J, et al. Inappropriate initial antimicrobial therapy and its effect on survival in a clinical trial of immunomodulating therapy for severe sepsis. Am J Med. 2003;115:529-535.
66. Ibrahim EH, Sherman G, Ward S, et al. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest. 2000;118:146-155.
67. Leibovici L, Shraga I, Drucker M, et al. The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection. J Intern Med. 1998;244:379-386.
68. Heyland DK, Dodek P, Muscedere J, et al. Randomized trial of combination versus monotherapy for the empiric treatment of suspected ventilator-associated pneumonia. Crit Care Med. 2008;36:737-744.
69. Paul M, Silbiger I, Grozinsky S, et al. Beta lactam antibiotic monotherapy versus beta lactam-aminoglycoside antibiotic combination therapy for sepsis. Cochrane Database Syst Rev. 2006;(1):CD003344.
70. Safdar N, Handelsman J, Maki DG. Does combination antimicrobial therapy reduce mortality in Gram-negative bacteraemia? A meta-analysis. Lancet Infect Dis. 2004;4:519-527.
71. Garnacho-Montero J, Sa-Borges M, Sole-Violan J, et al. Optimal management therapy for Pseudomonas aeruginosa ventilator-associated pneumonia: an observational, multicenter study comparing monotherapy with combination antibiotic therapy. Crit Care Med. 2007;35:1888-1895.
72. LeDoux D, Astiz ME, Carpati CM, Rackow EC. Effects of perfusion pressure on tissue perfusion in septic shock. Crit Care Med. 2000;28:2729-2732.
73. Kirchheim HR, Ehmke H, Hackenthal E, et al. Autoregulation of renal blood flow, glomerular filtration rate and renin release in conscious dogs. Pflugers Arch. 1987;410:441-449.
74. Bourgoin A, Leone M, Delmas A, et al. Increasing mean arterial pressure in patients with septic shock: effects on oxygen variables and renal function. Crit Care Med. 2005;33:780-786.
75. Martin C, Papazian L, Perrin G, et al. Norepinephrine or dopamine for the treatment of hyperdynamic septic shock? Chest. 1993;103:1826-1831.
76. Martin C, Viviand X, Leone M, Thirion X. Effect of norepinephrine on the outcome of septic shock. Crit Care Med. 2000;28:2758-2765.
77.www.clinicaltrials.gov. Identifier NCT00314704. Accessed January 13, 2009.
78. Annane D, Vignon P, Renault A, et al; CATS Study Group. Norepinephrine plus dobutamine versus epinephrine alone for the management of septic shock: a randomised trial [published correction appears in Lancet. 2007;370:1034]. Lancet. 2007;370:676-684.
79. Levy B, Bollaert PE, Charpentier C, et al. Comparison of norepinephrine and dobutamine to epinephrine for hemodynamics, lactate metabolism, and gastric tonometric variables in septic shock: a prospective, randomized study. Intensive Care Med. 1997;23:282-287.
80. Landry DW, Levin HR, Gallant EM, et al. Vasopressin pressor hypersensitivity in vasodilatory septic shock. Crit Care Med. 1997;25:1279-1282.
81. Landry DW, Levin HR, Gallant EM, et al. Vasopressin deficiency contributes to the vasodilation of septic shock. Circulation. 1997;95:1122-1125.
82. Holmes CL, Walley KR, Chittock DR, et al. The effects of vasopressin on hemodynamics and renal function in severe septic shock: a case series. Intensive Care Med. 2001;27:1416-1421.
83. Malay MB, Ashton RC Jr, Landry DW, Townsend RN. Low-dose vasopressin in the treatment of vasodilatory septic shock. J Trauma. 1999;47:699-703.
84. Patel BM, Chittock DR, Russell JA, Walley KR. Beneficial effects of short-term vasopressin infusion during severe septic shock. Anesthesiology. 2002;96:576-582.
85. Russell JA, Walley KR, Singer J, et al; VASST Investigators. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008;358:877-887.
86. Ely EW, Laterre PF, Angus DC, et al; PROWESS Investigators. Drotrecogin alfa (activated) administration across clinically important subgroups of patients with severe sepsis. Crit Care Med. 2003;31:12-19.
87. Abraham E, Laterre PF, Garg R, et al; Administration of Drotrecogin Alfa (Activated) in Early Stage Severe Sepsis (ADDRESS) Study Group. Drotrecogin alfa (activated) for adults with severe sepsis and a low risk of death. N Engl J Med. 2005;353:1332-1341.
88. Vincent JL, Bernard GR, Beale R, et al. Drotrecogin alfa (activated) treatment in severe sepsis from the global open-label trial ENHANCE: further evidence for survival and safety and implications for early treatment. Crit Care Med. 2005;33:2266-2277.
89. Levi M, Levy M, Williams MD, et al; Xigris and Prophylactic HepaRin Evaluation in Severe Sepsis (XPRESS) Study Group. Prophylactic heparin in patients with severe sepsis treated with drotrecogin alfa (activated). Am J Respir Crit Care Med. 2007;176:483-490.
90. Angus DC, Linde-Zwirble WT, Clermont G, et al; PROWESS Investigators. Cost-effectiveness of dro-trecogin alfa (activated) in the treatment of severe sepsis. Crit Care Med. 2003;31:1-11.
91. Green C, Dinnes J, Takeda A, et al. Clinical effectiveness and cost-effectiveness of drotrecogin alfa (activated) (Xigris) for the treatment of severe sepsis in adults: a systematic review and economic evaluation. Health Technol Assess. 2005;9(11):1-126, iii-iv.
92. Manns BJ, Lee H, Doig CJ, et al. An economic evaluation of activated protein C treatment for severe sepsis. N Engl J Med. 2002;347:993-1000.
93. Annane D, Bellissant E, Bollaert PE, et al. Corticosteroids for severe sepsis and septic shock: a systematic review and meta-analysis. BMJ. 2004;329:480.
94. Bone RC, Fisher CJ Jr, Clemmer TP, et al. A controlled clinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. N Engl J Med. 1987;317:653-658.
95. Cronin L, Cook DJ, Carlet J, et al. Corticosteroid treatment for sepsis: a critical appraisal and meta-analysis of the literature. Crit Care Med. 1995;23:1430-1439.
96. Sprung CL, Caralis PV, Marcial EH, et al. The effects of high-dose corticosteroids in patients with septic shock. A prospective, controlled study. N Engl J Med. 1984;311:1137-1143.
97. Annane D, Sébille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock [published correction appears in JAMA. 2008;300:1652]. JAMA. 2002;288:862-871.
98. Bollaert PE, Charpentier C, Levy B, et al. Reversal of late septic shock with supraphysiologic doses of hydrocortisone. Crit Care Med. 1998;26:645-650.
99. Briegel J, Forst H, Haller M, et al. Stress doses of hydrocortisone reverse hyperdynamic septic shock: a prospective, randomized, double-blind, single-center study. Crit Care Med. 1999;27:723-732.
100. Sprung CL, Annane D, Keh D, et al; CORTICUS Study Group. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358:111-124.
101. Vincent JL, Baron JF, Reinhart K, et al; ABC (Anemia and Blood Transfusion in Critical Care) Investigators. Anemia and blood transfusion in critically ill patients. JAMA. 2002;288:1499-1507.
102. Nguyen BV, Bota DP, Mélot C, Vincent JL. Time course of hemoglobin concentrations in nonbleeding intensive care unit patients. Crit Care Med. 2003;31:406-410.
103. Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group [published correction appears in N Engl J Med. 1999;340:1056]. N Engl J Med. 1999;340:409-417.
104. Claessens YE, Fontenay M, Pene F, et al. Erythropoiesis abnormalities contribute to early-onset anemia in patients with septic shock. Am J Respir Crit Care Med. 2006;174:51-57.
105. Rogiers P, Zhang H, Leeman M, et al. Erythropoietin response is blunted in critically ill patients. Intensive Care Med. 1997;23:159-162.
106. Corwin HL, Gettinger A, Rodriguez RM, et al. Efficacy of recombinant human erythropoietin in the critically ill patient: a randomized, double-blind, placebo-controlled trial. Crit Care Med. 1999;27:2346-2350.
107. Corwin HL, Gettinger A, Pearl RG, et al; EPO Critical Care Trials Group. Efficacy of recombinant human erythropoietin in critically ill patients: a randomized controlled trial. JAMA. 2002;288:2827-2835.
108. Corwin HL, Gettinger A, Fabian TC, et al; EPO Critical Care Trials Group. Efficacy and safety of epoetin alfa in critically ill patients. N Engl J Med. 2007;357:965-976.
