- Clinical Technology
- Adult Immunization
- Hepatology
- Pediatric Immunization
- Screening
- Psychiatry
- Allergy
- Women's Health
- Cardiology
- Pediatrics
- Dermatology
- Endocrinology
- Pain Management
- Gastroenterology
- Infectious Disease
- Obesity Medicine
- Rheumatology
- Nephrology
- Neurology
- Pulmonology
Drug-induced lung diseases: A state-of-the-art review
p>Drug-induced lung disease (DILD) can be caused by a variety of agents, including chemotherapeutic drugs, antiarrhythmic agents, antibiotics, and NSAIDs. The clinical syndromes associated with DILD include alveolar hypoventilation, acute bronchospasm, organizing pneumonia, and hypersensitivity reactions. Amiodarone lung toxicity often manifests as a chronic fibrosing alveolitis, characterized by an insidious onset of cough, dyspnea, and weight loss. Important components of the workup include chest radiography, pulmonary function testing, and bronchoscopy with bronchoalveolar lavage (BAL). BAL is particularly helpful in identifying eosinophilic pneumonia and diffuse alveolar hemorrhage and in ruling out infectious causes. Management includes drug withdrawal and, in some cases, corticosteroid therapy. Before starting corticosteroids, it is important to rule out infectious causes of lung disease, particularly in patients receiving chemotherapy. (J Respir Dis. 2009;30(1))
ABSTRACT: Drug-induced lung disease (DILD) can be caused by a variety of agents, including chemotherapeutic drugs, antiarrhythmic agents, antibiotics, and NSAIDs. The clinical syndromes associated with DILD include alveolar hypoventilation, acute bronchospasm, organizing pneumonia, and hypersensitivity reactions. Amiodarone lung toxicity often manifests as a chronic fibrosing alveolitis, characterized by an insidious onset of cough, dyspnea, and weight loss. Important components of the workup include chest radiography, pulmonary function testing, and bronchoscopy with bronchoalveolar lavage (BAL). BAL is particularly helpful in identifying eosinophilic pneumonia and diffuse alveolar hemorrhage and in ruling out infectious causes. Management includes drug withdrawal and, in some cases, corticosteroid therapy. Before starting corticosteroids, it is important to rule out infectious causes of lung disease, particularly in patients receiving chemotherapy. (J Respir Dis. 2009;30[1])
Osler first described heroin-induced pulmonary edema in 1880 during an autopsy.1,2 Since the late 19th century, a growing number of agents have been linked to pulmonary toxicity. Currently, more than 350 agents are associated with iatrogenic lung diseases.3,4
Drug-induced lung diseases (DILDs) vary in their pathophysiology, presentation, and prognosis. Proper diagnosis requires a high index of suspicion and familiarity with the clinical syndromes associated with DILD. Most cases of pulmonary toxicity are idiosyncratic, and literature searches yield only small numbers of case reports for a given exposure.
Therapies for DILD are usually recommended on the basis of expert opinion rather than on well-designed clinical studies. Moreover, DILD is typically a diagnosis of exclusion. Therefore, before starting adjuvant therapies, such as corticosteroids, for presumed DILD, alternative causes of respiratory illness, such as infection, must be excluded.
DILD represents a heterogeneous group of diseases that, at least initially, is an imposing field to master. However, in this review, we hope to organize and clarify the myriad of lung diseases. We will limit our discussion to the most common DILDs encountered by health care professionals. We will first describe the typical clinical syndromes and diagnostic tests and then review the common agents associated with pulmonary toxicity, including chemotherapeutic drugs, antiarrhythmic drugs, antibiotics, anticonvulsants, anti-inflammatory agents, and illicit drugs.
CLINICAL SYNDROMES
Several clinical syndromes are associated with pulmonary toxicity. Organizing the vast number of potentially toxic drugs by clinical syndromes allows the health professional to link clinical presentations with the likely drug toxicity. The syndromes most often encountered and examples of the offending agents are listed in the Table.5
Alveolar hypoventilation
This form of DILD does not directly affect the lung parenchyma. Alveolar hypoventilation results from decreased ventilatory function resulting in increased arterial concentration of carbon dioxide, often with concomitant hypoxemia. If alveolar hypoventilation occurs by itself, chest radiography does not demonstrate pulmonary infiltrates, but it may show decreased lung volumes. In this setting, arterial blood gas analysis usually reveals a normal alveolar-arterial gradient.
Hypoventilation is typically associated with over-sedation from narcotics with resultant decreased ventilatory drive. However, agents that cause neuromuscular blockade or myopathy may also lead to alveolar hypoventilation. Both directly affect neuromuscular strength leading to hypoventilation. γ-Hydroxybutyrate and aminogylcosides are known to induce neuromuscular blockade, while corticosteroids can cause myopathy.
Acute bronchospasm
This form of airflow limitation is typically seen shortly after drug administration and manifests as wheezing and shortness of breath. As with alveolar hypoventilation, chest radiographs do not show infiltrates but, in the case of bronchospasm, may show hyperinflation. Acute bronchospasm can be triggered via any route of drug delivery including aerosol, oral, or intravenous.
Common causes of drug-induced acute bronchospasm include NSAIDs and β-blockers. Acute bronchospasm with β-blockers is more likely to be seen in patients with asthma and patients with chronic obstructive pulmonary disease (COPD) who have a component of reversible airflow limitation (bronchodilator response). However, in one study, patients with coronary artery disease and COPD benefited from β-blockers; thus, systematic avoidance of these agents in patients with COPD appears to be unwarranted.6,7
Bronchiolitis obliterans
This is a chronic form of airflow limitation characterized by inflammation and eventual occlusion of the small airways.8 Bronchiolitis obliterans usually presents as subacute or chronic shortness of breath and wheezing. Findings may include decreased forced expiratory volume in 1 second (FEV1) and forced expiratory flow at 25% to 75% of forced vital capacity (FVC) (FEF25-75) and hyperinflation on a chest radiograph.9 Medications implicated in bronchiolitis obliterans include cyclophosphamide, methotrexate, CCNU, and penicillamine.8
Noncardiogenic pulmonary edema
This manifests as the simultaneous presence of hypoxemia and bilateral alveolar infiltrates in the absence of left-sided heart dysfunction. The exact mechanism of noncardiogenic pulmonary edema is not well understood, but increased alveolar capillary membrane permeability has been suggested. Increased permeability allows protein and fluid to enter the lung interstitium and then flood the alveolar spaces. Drugs implicated in noncardiogenic pulmonary edema include narcotics, salicylates, tocolytics, hydrochlorothiazide, and protamine.10
Hypersensitivity-type lung disease
This can be caused by virtually any drug and is associated with pulmonary infiltrates and eosinophilia (PIE). Patients may acutely present with Lffler syndrome, which is characterized by cough, dyspnea, fever, rash, blood eosinophilia, and fleeting pulmonary infiltrates.
Another form of PIE syndrome is chronic eosinophilic pneumonia. Patients present with subacute low-grade fever, weight loss, and nonproductive cough. Chest radiographs demonstrate patchy peripheral airspace consolidation. Bronchoscopy with bronchoalveolar lavage (BAL) can confirm the diagnosis of PIE by revealing eosinophilia (more than 25% eosinophils). Antibiotics such as β-lactams as well as sulfa-containing drugs are common examples of agents that may induce Lffler syndrome and chronic eosinophilic pneumonia.11
Drug-induced hypersensitivity pneumonitis is a clinically distinct pulmonary syndrome characterized by a complex immunological reaction.12 Patients may present with acute, subacute, or chronic symptoms of fevers, chills, malaise, and dyspnea. Pulmonary function tests demonstrate a reduced FVC and carbon monoxide–diffusing capacity (DLCO). High-resolution CT (HRCT) of the chest may reveal bilateral upper lobe–predominant ground-glass opacities, poorly defined centrilobular nodules, and air trapping on expiratory scans (Figure 1).
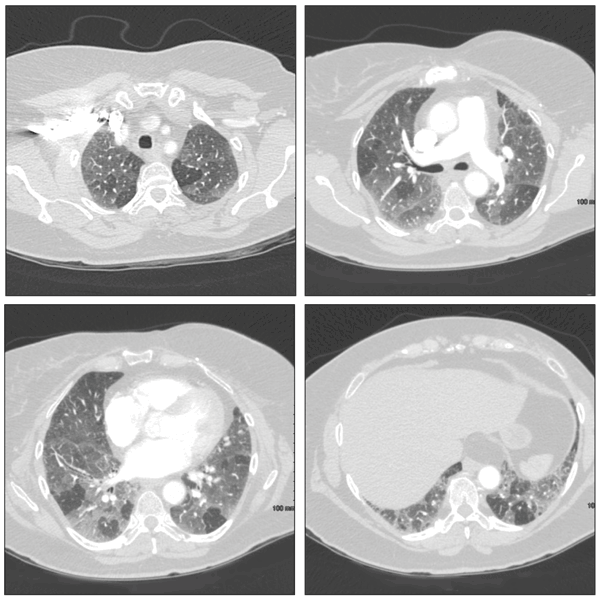
Figure 1 – These CT scans are from a patient with a subacute hypersensitivity reaction to nitrofurantoin. Findings include bilateral patchy areas of ground-glass opacity and areas of air trapping.
In chronic cases, radiographic and HRCT features may progress to fibrotic changes, including honeycombing and traction bronchiectasis (Figure 2).13 Lung biopsy specimens demonstrate loosely formed granulomas near the terminal bronchioles and lymphocytic and plasma cell infiltration of the alveolar walls (Figure 3). Pathology may demonstrate a nonspecific or usual interstitial pneumonitis pattern.13
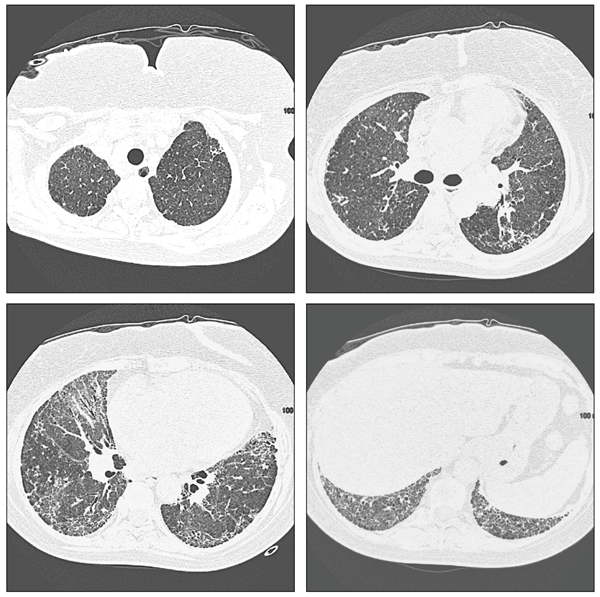
Figure 2 – Examination of the lung parenchyma demonstrates diffuse ground-glass opacities and fibrotic changes, including areas of reticulation and honeycombing, which are worse at the periphery and the lung bases. Scattered areas of dilated airways are consistent with traction bronchiectasis.
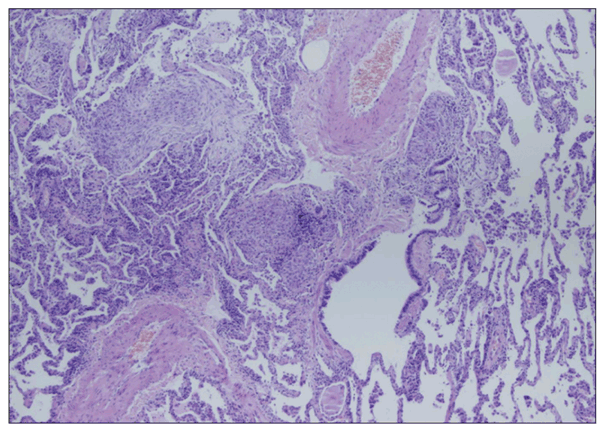
Figure 3 – Chronic interstitial pneumonia, loose granulomas, and areas of organizing pneumonia are consistent with hypersensitivity pneumonitis.
Drug-induced hypersensitivity pneumonitis typically responds to drug withdrawal alone, but in cases that are slow to respond, corticosteroid therapy may be helpful, with an initial prednisone dosage of 0.5 to 1 mg/kg/d, followed by a prolonged taper. Common examples of drugs associated with a hypersensitivity pneumonitis include nitrofurantoin and methotrexate.14
Organizing pneumonia
Previously known as drug-induced bronchiolitis obliterans with organizing pneumonia, drug-induced organizing pneumonia is a unique form of lung disease that often requires lung biopsy for diagnosis.15 Bronchiolitis obliterans is no longer considered a major feature of the disease and now is known as organizing pneumonia.15
Patients with organizing pneumonia may present with a flu-like illness with fever, cough, weight loss, and progressive dyspnea. Chest radiographs demonstrate patchy areas of consolidation, ground-glass and nodular opacities, and bronchial wall thickening and dilation (Figure 4).16 Lung biopsy demonstrates preserved lung architecture with distinct intraluminal buds of granulation tissue (Figure 5).
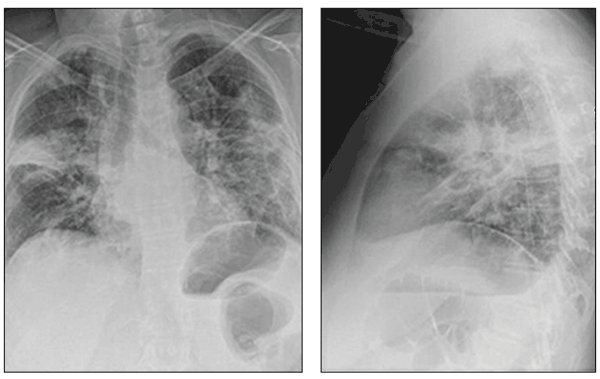
Figure 4 – Multifocal airspace opacities can be seen on these chest radiographs from a patient with drug-induced organizing pneumonia.
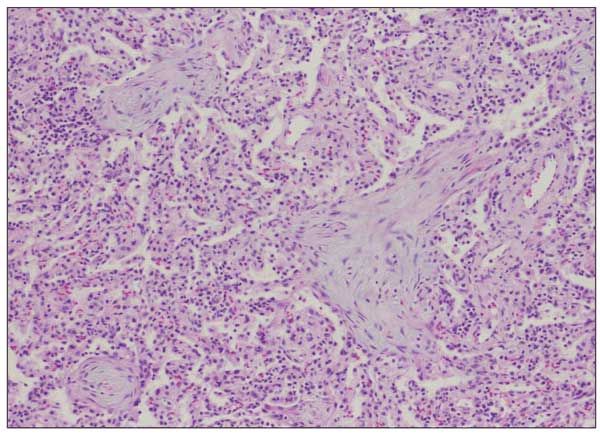
Figure 5 – Plugs of loose fibromyxoid tissue that fill the alveolar ducts and alveoli are consistent with organizing pneumonia.
Drug-induced organizing pneumonia responds to drug withdrawal and corticosteroid therapy, with an initial dosage of 0.75 to 1.5 mg/kg/d of methylprednisolone, followed by a taper.15 Examples of agents associated with drug-induced organizing pneumonia include amiodarone, bleomycin, and carbamazepine.17
Alveolitis
Alveolitis with or without pulmonary fibrosis is a well-recognized clinical syndrome. Patients with alveolitis typically present with shortness of breath that may be acute, subacute, or chronic. Features on physical examination include inspiratory lung crackles, weight loss, and sometimes clubbing of the digits.
HRCT scans may reveal early symmetric reticular interstitial markings progressing to fibrotic changes, including honeycombing and traction bronchiectasis. Pulmonary function tests demonstrate restrictive changes with reduced FVC and total lung capacity (TLC), as well as decreased DLCO. Bleomycin is the most common chemotherapeutic agent implicated in alveolitis, while amiodarone is the most common nonchemotherapeutic agent.18
Drug-induced lupus erythematosus
In drug-induced systemic lupus erythematosus, reactions are signaled by the development of positive antinuclear antibodies (ANAs) and/or antihistone antibodies. Patients may complain of dyspnea, pleurisy, fevers, rash, arthralgias, and joint swelling. Chest radiographs may reveal bilateral infiltrates and/or pleural effusions.
The most common agents implicated in drug-induced lupus erythematosus reactions are hydralazine, procainamide, quinidine, isoniazid, and penicillamine.19
Alveolar hemorrhage
Patients with alveolar hemorrhage present with dyspnea, bilateral infiltrates, new-onset anemia, and sometimes hemoptysis. The formal diagnosis of alveolar hemorrhage requires bronchoscopy in which serial BAL samples reveal increasingly hemorrhagic fluid.
Drugs that may induce alveolar hemorrhage include oral anticoagulants, such as coumadin, aspirin, fibrinolytics, and platelet glycoprotein inhibitors. Other drugs that have been reported to cause diffuse alveolar hemorrhage include amiodarone, sirolimus,20 and crack cocaine.21
DIAGNOSTIC TESTS
The diagnostic approach to DILD requires careful review of the patient’s presentation and medical history with meticulous attention to current and past medication use, including dosages and schedule. Establishing a temporal relationship between the administration of a drug and the development of lung disease is the first step toward determining whether the patient may have pulmonary toxicity.
There are no definitive physical examination, laboratory, or radiographic features of the clinical syndromes associated with DILD. At initial presentation, plain chest radiographs are likely to miss or underestimate the presence of lung disease. Therefore, HRCT may be helpful in the initial workup.
Pulmonary function testing is useful in supporting the diagnosis of pulmonary toxicity. Evidence of fibrosing alveolitis includes a restrictive pattern with a decreased FVC and TLC, as well as a decreased DLCO. Acute bronchospasm is characterized by obstructive changes, including a reduced FEV1 and FEV1:FVC ratio, often with a significant bronchodilator response. Bronchiolitis obliterans may be suggested by evidence of fixed obstructive lung disease, initially manifested by a reduced FEF1, followed by decreasing FEV25-75 with a low FEV1:FVC ratio without a bronchodilator response.
Other noninvasive diagnostic measures may help guide the diagnostic workup. Complete blood cell count with differential can detect eosinophilia. The presence of blood eosinophilia may suggest Lffler or DRESS (drug rash with eosinophilia and systemic symptoms) syndrome. It is important to note that the concomitant use of corticosteroids may mask this. In the appropriate setting, echocardiography may help rule out cardiac disease, sputum studies may identify an infectious cause, and immunological serological findings such as a rheumatoid factor or ANAs may be helpful in the workup of a suspected collagen-vascular disease.
If a diagnosis is not obtained by noninvasive measures, more invasive tests, including fiberoptic bronchoscopy with BAL, may be indicated. BAL is particularly helpful in identifying eosinophilic pneumonia (more than 25% eosinophils) and diffuse alveolar hemorrhage (increasingly bloody return during serial lavage instillations). Bronchoscopy with negative cytopathological and culture results allows the physician to rule out with a fair degree of certainty infectious causes, which may be important not only to the establishment of DILD but also to the empirical use of corticosteroid therapy.
Video-assisted thoracic surgery is likely to offer more definitive information but may demonstrate nonspecific histopathological changes, such as type 2 pneumocyte hyperplasia, collagen deposits, inflammatory cells, or end-stage fibrotic changes. Biopsy is especially helpful in diagnosing bronchiolitis obliterans, organizing pneumonia, and hypersensitivity pneumonitis.
DRUGS THAT MAY CAUSE DILD
Chemotherapeutic agents
Many drugs used in the treatment of hematological malignancies and solid organ tumors have the potential to save or extend lives; unfortunately, some of these chemotherapeutic agents can cause devastating lung toxicity.
• Bleomycin: This antitumor drug is derived from Streptomyces verticillus and induces cell death through the formation of breaks in cellular DNA. Bleomycin is associated with several clinically distinct syndromes, including bleomycin-induced pneumonitis (BIP), bleomycin hypersensitivity syndrome, and bleomycin-induced organizing pneumonia.
The most common presentation of bleomycin toxicity is pneumonitis. It usually develops during therapy but can present up to 6 months after discontinuation of the drug. Patients present with dyspnea, inspiratory crackles and, often, a nonproductive cough.22 Early changes on HRCT scans include small linear and subpleural nodules at the lung bases, which may progress to diffuse bilateral fibrosis and honeycombing. Lung biopsy specimens reveal nonspecific findings, including squamous metaplasia of bronchiolar epithelium, alveolar inflammatory cells, collagen deposition, and fibrosis.23,24
The risk of BIP is associated with the cumulative dose of bleomycin. The incidence of BIP is 3% to 5% in patients treated with 300 mg of bleomycin and increases to 20% in patients receiving 500 mg or more.25 Bleomycin is renally excreted, and renal insufficiency also increases the risk of BIP. Radiation therapy, which is often combined with bleomycin therapy, is thought to have a synergistic effect on the development of BIP.
BIP has also been linked to concomitant exposure to high oxygen tension. It is theorized that high fractional inspired oxygen potentiates bleomycin lung toxicity through free radical formation; however, evidence is limited to experimental rat models, and its role in human disease is unclear.26
Bleomycin hypersensitivity syndrome is a relatively rare complication, characterized by blood and pulmonary eosinophilia, similar to drug-induced acute eosinophilic pneumonia. Bleomycin-induced organizing pneumonia is characterized by fever with migratory infiltrates on chest radiographs and often requires lung biopsy for confirmation.
As with all DILD, drug withdrawal is the primary therapeutic approach. Corticosteroid therapy may be considered for BIP, bleomycin hypersensitivity syndrome, and bleomycin-induced organizing pneumonia. The initial dosage of prednisone is 0.5 to 1 mg/kg/d and should be slowly tapered. It is important to rule out infectious causes of lung disease before starting corticosteroids, particularly in patients receiving chemotherapy. This is typically accomplished with fiberoptic bronchoscopy and BAL.
• Mitomycin C: This is an antibiotic with antitumor activity that is derived from Streptomyces caespitosus and has been associated with 2 distinct toxicity syndromes: mitomycin C–induced pulmonary fibrosis and mitomycin C/vinca alkaloids acute bronchospasm and pulmonary infiltrates.
Mitomycin C–induced pulmonary fibrosis occurs in 2% to 12% of patients, usually 6 to 12 months after therapy is completed. Risk factors include high concentrations of inspired oxygen, prior radiation exposure, and cumulative doses of mitomycin greater than 10 mg/m2.5 Clinical findings include nonproductive cough, dyspnea, and bilateral infiltrates with focal areas of consolidation. Pulmonary function tests demonstrate evidence of restriction with a decreased DLCO.
When given in combination with vinca alkaloids, mitomycin C may be associated with the development of acute bronchospasm and pulmonary infiltrates.27,28 The pathophysiological mechanisms underlying this syndrome are unknown. This syndrome may occur in more than 5% of treated patients. Typically, patients have a sudden onset of dyspnea and wheezing occurring up to 4 hours after the initiation of drug therapy. Patients may have progressive hypoxemia, and respiratory failure requiring mechanical ventilation occurs in 20% of cases.
Pulmonary function tests demonstrate airflow limitation during the acute phase, and diffuse bilateral infiltrates may be present on chest radiographs. The symptoms usually resolve within 24 hours after drug withdrawal, and treatment is primarily supportive.
• Cyclophosphamide: This alkylating cytotoxic agent is used in the treatment of a variety of malignancies, including lymphoma and solid tumors. It is also used in the treatment of vasculitis and interstitial lung disease. Cyclophosphamide-induced lung disease is temporally divided into early- and late-onset fibrosing alveolitis syndromes. Both syndromes are characterized by restrictive changes and a decreased DLCO on pulmonary function tests; on HRCT scans, diffuse reticular or reticulonodular patterns of fibrosis are common.5
Early-onset cyclophosphamide lung toxicity develops 1 to 6 months after drug exposure. The clinical presentation includes dyspnea, cough, and fever, and patients may have a rapid clinical deterioration. Lung biopsy reveals a mixture of red blood cells, polymorphonuclear cells, and histiocytes infiltrating the interstitial and alveolar spaces. Early-onset cyclophosphamide lung toxicity is typically corticosteroid-responsive.29
Late-onset cyclophosphamide lung toxicity occurs one to several years after the administration of the drug and does not respond to corticosteroids. Pleural thickening and pneumothorax are frequent complications. Pathology reveals type II alveolar hyperplasia, interstitial collagen, fibrosis, and honeycombing. Progressive pulmonary fibrosis is common, with a mortality rate of more than 60%.29,30
• All-trans retinoic acid (ATRA): This is a chemotherapeutic agent specifically used for acute promyelocytic leukemia. ATRA induces differentiation of leukemic blasts into mature granulocytes. These mature granulocytes appear to adhere to the pulmonary endothelium and can cause diffuse lung toxicity.
ATRA syndrome is characterized by fever, shortness of breath, and radiographic findings of diffuse pulmonary infiltrates with small irregular peripheral nodules, patchy ground-glass opacities, and pleural effusions developing within 10 days of ATRA treatment. In addition to pulmonary symptoms, some patients may have pericardial effusion, hypotension, and renal failure.
When ATRA syndrome is suspected, in addition to the withdrawal of ATRA, high-dose dexamethasone, 10 mg intravenously every 12 hours, should be given for 3 or more days.31 Interestingly, once symptoms resolve, ATRA therapy can be restarted in most patients.32 Mortality from ATRA syndrome ranges from 7% to 28% in the literature; however, early use of induction chemotherapy with prophylactic dexamethasone has reduced the risk of mortality to approximately 1%.
• Methotrexate: Methotrexate is used in the treatment of many chronic conditions, including rheumatoid arthritis, psoriasis, and primary biliary cirrhosis. Acute methotrexate pneumonitis occurs in up to 11% of patients, and chest radiographs typically demonstrate bilateral alveolar and/or interstitial infiltrates. Risk factors include higher cumulative doses (more than 20 mg/wk), renal insufficiency, and concomitant treatment with aspirin or NSAIDs.
Patients receiving methotrexate therapy are at increased risk for opportunistic infections, particularly Pneumocystis jiroveci pneumonia, which should be ruled out with induced sputum with hypertonic saline and/or bronchoscopy with BAL before establishing a diagnosis of methotrexate-induced lung disease.33,34 This form of DILD can also present as a granulomatous process similar to sarcoidosis. In addition to methotrexate discontinuation, affected patients may respond to corticosteroid treatment, usually 0.5 to 1 mg/kg/d initially, followed by a slow taper.35
Antiarrhythmic agents
Since patients receiving antiarrhythmic agents often have underlying cardiac disease, differentiating between shortness of breath caused by heart failure or underlying pulmonary drug toxicity can be a challenge. We will limit our discussion to the most common antiarrhythmic agent–induced lung disease, amiodarone lung toxicity. However, it is important to note that drug-induced lupus reactions may be associated with antiarrhythmic agents, such as procainamide and quinidine, and this possibility should be considered in patients receiving these agents who experience lupus-like syndromes.
• Amiodarone: Pulmonary toxicity occurs in approximately 5% of patients receiving amiodarone, and the associated mortality rates are 10% to 20%. Amiodarone is an iodine-based derivative known to have many long-term, multiorgan sequelae. Compounding its known risk of toxicity, amiodarone has a high volume of distribution with an elimination half-life of 30 to 60 days, and thus blood levels of amiodarone may persist for weeks to months after discontinuation.36,37
Amiodarone lung toxicity may manifest as an acute/subacute alveolitis that imitates acute respiratory distress syndrome (ARDS), but it more commonly manifests as a chronic fibrosing alveolitis, usually characterized by an insidious onset of cough, dyspnea, and weight loss.38 Annual pulmonary function testing is helpful for detecting restrictive changes and decreased DLCO, which characterize the chronic form of amiodarone lung toxicity.
Chest radiographs tend to miss or underestimate the presence of amiodarone DILD. HRCT scanning without contrast is significantly more sensitive in detecting this disease. Radiographic findings include bilateral or mixed interstitial and alveolar infiltrates. Rarely, patients may have isolated pleural effusions or pleural-based lesions, solitary pulmonary nodules, or lobar infiltrates.
If BAL is performed, results may demonstrate a lymphocytosis. The most common finding is the appearance of foamy alveolar macrophages. However, this occurs in almost all patients taking amiodarone, including those without pulmonary toxicity, so this finding is not helpful in diagnosing amiodarone-induced lung disease.
Histologically, amiodarone toxicity presents in several forms, including acute findings similar to those of ARDS (diffuse alveolar damage) and a chronic fibrosing alveolitis. Acutely, there is evidence of intra-alveolar hemorrhage, type II alveolar epithelial cell proliferation, and hyaline membrane formation. Chronic disease is characterized by septal thickening with infiltration of lymphocytes, monocytes, and plasma cells.
The management of amiodarone-induced lung disease involves excluding alternative causes and switching antiarrhythmic agents. Corticosteroid therapy may be beneficial.
Antibiotics
Antibiotic-induced lung diseases vary in their presentation but are associated with both acute and chronic hypersensitivity reactions. Nitrofurantoin deserves special attention as a potential cause of DILD because it is the antibiotic most commonly associated with pulmonary toxicity.
• Nitrofurantoin: Two clinically distinct syndromes are associated with nitrofurantoin exposure: acute hypersensitivity reaction and chronic pneumonitis. Acute nitrofurantoin lung toxicity manifests as dyspnea, pleurisy, cough, fever and, sometimes, a macular-papular rash. An elevated erythrocyte sedimentation rate and blood eosinophilia are often present.
Patients typically have a mixed alveolar and interstitial pattern on chest radiographs. About one-third of patients with nitrofurantoin toxicity may also present with small pleural effusions. Pulmonary function tests show a restrictive pattern with a decreased DLCO. Patients frequently do well with withdrawal of the drug alone, but in those with slow improvement, corticosteroid therapy may be helpful.39
Chronic nitrofurantoin lung toxicity is a form of hypersensitivity reaction that occurs primarily in patients treated with long-term nitrofurantoin therapy for prophylaxis of recurrent urinary tract infections.40 This form of chronic lung toxicity is rare, but patients may have long-standing debilitating respiratory complications. Patients may have an insidious onset of dyspnea, fatigue, and weight loss, and a positive ANA or rheumatoid factor finding. Chest radiographs typically demonstrate bilateral interstitial infiltrates and, with time, pulmonary fibrosis may occur.
Lung biopsy specimens demonstrate lymphocytic interstitial inflammation with fibrosis. In the latter stages of chronic nitrofurantoin lung toxicity, the histological findings may be indistinguishable from those of idiopathic pulmonary fibrosis. Most patients with chronic nitrofurantoin lung toxicity fail to improve despite drug withdrawal and corticosteroid therapy. The overall mortality rate is 8% to 10%.5
Antibiotic-induced hypersensitivity type lung diseases
Antibiotic-induced hypersensitivity type lung diseases present as PIE syndromes, including Lffler syndrome and chronic eosinophilic pneumonia.3 Lffler syndrome manifests as the acute onset of cough, dyspnea, fever, rash, blood eosinophilia, and transient peripheral lung infiltrates.
Chronic eosinophilic pneumonia is characterized by a subacute presentation over weeks to months, with a low-grade fever, night sweats, nonproductive cough, and weight loss. Patients typically have migratory bilateral peripheral pulmonary infiltrates, and the diagnosis is made by examination of BAL fluid, which reveals eosinophilia.
Patients with chronic eosinophilic pneumonia typically respond to drug withdrawal alone, but oral prednisone is helpful.41 Patients should receive 0.5 to 1 mg/kg/d of prednisone initially until there is radiographic improvement, then prednisone is tapered slowly.
Common agents that have been associated with PIE syndromes include β-lactam antibiotics (especially penicillins and cephalosporins) and sulfa-containing medications, including dapsone, trimethoprim/sulfamethoxazole, sulfonamides, sulfadoxine, and sulfasalazine.
Anticonvulsants
Phenytoin is a commonly used antiseizure medication that may be associated with a unique DILD syndrome. Phenytoin-hypersensitivity reaction is a relatively rare DILD, with an estimated incidence of 1 in 1000 to 1 in 10,000 exposures. The syndrome may also be described as DRESS syndrome. DRESS syndrome is linked in some patients to a genetic deficiency in the enzyme epoxide hydrolase. Patients with epoxide hydrolase deficiency are unable to detoxify metabolic intermediates of phenytoin.42
Fever, lymphadenopathy, peripheral eosinophilia, and a diffuse rash typically develop about 1 month after starting phenytoin therapy. Severe systemic complications, including renal failure, hepatitis, myalgias/arthralgias, hematological abnormalities, periorbital edema, aseptic meningitis, and hypoxic respiratory failure, may also develop.43
Radiographs demonstrate a mixed alveolar and interstitial pattern of infiltrates. Bronchoscopy and BAL reveal lymphocytic and eosinophilic predominance, while lung biopsy may reveal capillaritis.42 Treatment consists of drug withdrawal and supportive measures.
Anti-inflammatory agents
Commonly used anti-inflammatory agents, such as NSAIDs and aspirin, are often associated with DILD. Aspirin-induced bronchospasm occurs in 1% of persons without asthma and up to 20% of those with asthma. Samter and Beers first described a clinical syndrome known as Samter’s triad, which includes asthma, aspirin sensitivity, and nasal polyps.44 Symptoms can occur within minutes to hours after aspirin ingestion and include angioedema, facial flushing, and conjunctivitis.
Aspirin and NSAIDs both induce acute bronchospasm by increasing production of leukotrienes via the 5-lipoxygenase pathway with cyclooxygenase inhibition.3 Agents that do not block the cyclooxygenase pathway, such as acetaminophen, can be safely used in patients with aspirin/NSAID-induced bronchospasm. In addition to avoidance, therapy may include a lipoxygenase inhibitor, zileuton, and/or a leukotriene receptor antagonist, zafirlukast or montelukast.3 Rarely, desensitization may be considered.
Another form of DILD is aspirin-induced noncardiogenic pulmonary edema, which is associated with long-term exposure and resolves with withdrawal of the medication.
NSAIDs are among the most commonly used over-the-counter medications and have been associated with both acute hypersensitivity and acute bronchospasm syndromes. NSAID hypersensitivity pneumonitis typically develops within 1 week of exposure but may develop several years later. Patients present with cough, fever, dyspnea, rash, and blood eosinophilia. Chest radiographs may reveal bilateral infiltrates or patchy peripheral infiltrates with a “radiographic negative of pulmonary edema.”5 The symptoms typically resolve after drug withdrawal.
NSAID-induced acute bronchospasm occurs through the inhibition of cyclooxygenase and an increase in leukotrienes via the 5-lipoxygenase pathway, a mechanism that is similar to that of aspirin-induced bronchospasm. Patients with NSAID-induced hypersensitivity reaction or bronchospasm improve after the withdrawal of NSAIDs.
Illicit drugs
Since Osler first described heroin-induced noncardiogenic pulmonary edema during an autopsy in 1880, illicit drugs have been implicated in DILD. We will specifically discuss DILD related to cocaine, opiates, and talc, a common contaminant of injectable drugs of abuse.
• Cocaine: While cocaine hydrochloride can be snorted or injected, it is not heat-stable and cannot be smoked. The advent of “free-based” or crack cocaine in the early 1980s provided a heat-stable form of cocaine that can readily be inhaled. “Crack” refers to the popping sound made during the manufacturing process, and this form offers a more intense “high” than powdered cocaine.
Crack cocaine lung disease, or “crack lung,” is a distinct clinical syndrome. Symptoms include pleurisy, cough, wheezing, dyspnea, hemoptysis, and sometimes upper airway thermal burns. Rare complications include alveolar hemorrhage, pneumothorax, pneumomediastinum, and pneumopericardium.45 Unlike tobacco, crack cocaine does not appear to affect DLCO.46
Other findings in patients who use crack cocaine include increased levels of iron and ferritin in BAL fluid, which may contribute to chronic lung disease. The exact mechanism of increased iron and ferritin levels-whether it is recurrent low-grade alveolar hemorrhage or increased capillary permeability-is not clear. Nonetheless, the increased iron and ferritin levels alter bactericidal activity by changing macrophage function and cell cytokine expression.21,45
Cocaine-induced pulmonary edema may also occur. This is often difficult to differentiate from cocaine-induced myocardial infarction with cardiogenic pulmonary edema, but patients typically recover within 1 to 2 days.
Other manifestations of cocaine-induced lung disease include alveolar hemorrhage and an acute hypersensitivity reaction with several days of cough, dyspnea, fever, rash, blood eosinophilia, and fleeting pulmonary infiltrates.
• Opiates: Besides the more common syndrome of alveolar hypoventilation, opiates may cause pulmonary edema up to 24 hours after the initial dose. Opiate intoxication is often accompanied by an aspiration pneumonitis or pneumonia. If aspiration is absent or mild, pulmonary edema may resolve within 72 hours.
Talc pulmonary granulomatosis is associated with the use of talc or hydrous magnesium silicate in the preparation of illicit injectable drugs, particularly heroin, or the use of oral opioids, such as methadone, in homemade injectable preparations. Afflicted patients present with cough, shortness of breath, and sometime hemoptysis.
After injection, drugs “cut” with talc induce a diffuse pulmonary reaction that is characterized radiographically by reticulonodular infiltrates or, rarely, progressive massive fibrosis similar to a pneumoconiosis. Lung biopsy may help confirm the diagnosis by demonstrating noncaseating granulomatous disease with birefringent talc particles.5 Therapy for pulmonary talc granulomatosis includes oral corticosteroid therapy and/or inhaled high-dose corticosteroids.47
CONCLUSION
DILD represents a heterogeneous group of diseases with protean manifestations. Clinicians should maintain a high index of suspicion for the possibility of this diagnosis and be aware of associated clinical syndromes. With this, a low threshold should exist to obtaining appropriate chest imaging and other investigations, and an aggressive approach should be taken to rule out alternative causes.
An excellent Internet resource is www.pneumotox.com.11 The Web site organizes DILDs by clinical or radiographic presentation and causative drugs. It is a current compilation of the ever-increasing number of DILDs.
References:
1. Sternbach G. William Osler: narcotic-induced pulmonary edema. J Emerg Med. 1983;1:165-167.
2. Sporer KA, Dorn E. Heroin-related noncardiogenic pulmonary edema: a case series. Chest. 2001;120:1628-1632.
3. Ozkan M, Dweik RA, Ahmad M. Drug-induced lung disease. Cleve Clin J Med. 2001;68:782-785, 789-795.
4. Rosenow EC 3rd. Drug-induced pulmonary disease. Dis Mon. 1994;40:253-310.
5. Schwarz MI, King TE. Interstitial Lung Disease. 4th ed. Hamilton, Ontario; Lewiston, NY: BC Decker; 2003.
6. Salpeter S, Ormiston T, Salpeter E. Cardioselective beta-blocker use in patients with reversible airway disease. Cochrane Database Syst Rev. 2001;(2):CD002992.
7. Au DH, Bryson CL, Fan VS, et al. Beta-blockers as single-agent therapy for hypertension and the risk of mortality among patients with chronic obstructive pulmonary disease. Am J Med. 2004;117:925-931.
8. Visscher DW, Myers JL. Bronchiolitis: the pathologist’s perspective. Proc Am Thorac Soc. 2006;3:41-47.
9. King TE Jr. Overview of bronchiolitis. Clin Chest Med. 1993;14:607-610.
10. Reed CR, Glauser FL. Drug-induced noncardiogenic pulmonary edema. Chest. 1991;100:1120-1124.
11. Camus P, Fanton A, Bonniaud P, et al. Interstitial lung disease induced by drugs and radiation. Respiration. 2004;71:301-326.
12. Bourke SJ, Dalphin JC, Boyd G, et al. Hypersensitivity pneumonitis: current concepts. Eur Respir J Suppl. 2001;32:81S-92S.
13. Silva CI, Churg A, Muller NL. Hypersensitivity pneumonitis: spectrum of high-resolution CT and pathologic findings. AJR. 2007;188:334-344.
14. Louie S, Lillington GA. Low dose methotrexate pneumonitis in rheumatoid arthritis. Thorax. 1986;41:703-704.
15. Cordier JF. Cryptogenic organising pneumonia. Eur Respir J. 2006;28:422-446.
16. Ujita M, Renzoni EA, Veeraraghavan S, et al. Organizing pneumonia: perilobular pattern at thin-section CT. Radiology. 2004;232:757-761.
17. Epler GR. Drug-induced bronchiolitis obliterans organizing pneumonia. Clin Chest Med. 2004;25:89-94.
18. Camus P, Bonniaud P, Fanton A, et al. Drug-induced and iatrogenic infiltrative lung disease. Clin Chest Med. 2004;25:479-519, vi.
19. Birnbaum B, Sidhu GS, Smith RL, et al. Fulminating hydralazine-induced lupus pneumonitis. Arthritis Rheum. 2006;55:501-506.
20. Vlahakis NE, Rickman OB, Morgenthaler T. Sirolimus-associated diffuse alveolar hemorrhage. Mayo Clin Proc. 2004;79:541-545.
21. Janjua TM, Bohan AE, Wesselius LJ. Increased lower respiratory tract iron concentrations in alkaloidal (“crack”) cocaine users. Chest. 2001;119:422-427.
22. White DA, Stover DE. Severe bleomycin-induced pneumonitis. Clinical features and response to corticosteroids. Chest. 1984;86:723-728.
23. Jones AW. Bleomycin lung damage: the pathology and nature of the lesion. Br J Dis Chest. 1978;72:321-326.
24. Bedrossian CW, Luna MA, Mackay B, Lichtiger B. Ultrastructure of pulmonary bleomycin toxicity. Cancer. 1973;32:44-51.
25. Collis CH. Lung damage from cytotoxic drugs. Cancer Chemother Pharmacol. 1980;4:17-27.
26. Sleijfer S. Bleomycin-induced pneumonitis. Chest. 2001;120:617-624.
27. Luedke D, McLaughlin TT, Daughaday C, et al. Mitomycin C and vindesine associated pulmonary toxicity with variable clinical expression. Cancer. 1985;55:542-545.
28. Rivera MP, Kris MG, Gralla RJ, White DA. Syndrome of acute dyspnea related to combined mitomycin plus vinca alkaloid chemotherapy. Am J Clin Oncol. 1995;18:245-250.
29. Malik SW, Myers JL, DeRemee RA, Specks U. Lung toxicity associated with cyclophosphamide use. Two distinct patterns. Am J Respir Crit Care Med. 1996;154(6 pt 1):1851-1856.
30. Hamada K, Nagai S, Kitaichi M, et al. Cyclophosphamide-induced late-onset lung disease. Intern Med. 2003;42:82-87.
31. Frankel SR, Eardley A, Lauwers G, et al. The “retinoic acid syndrome” in acute promyelocytic leukemia. Ann Intern Med. 1992;117:292-296.
32. Tallman MS, Andersen JW, Schiffer CA, et al. Clinical description of 44 patients with acute promyelocytic leukemia who developed the retinoic acid syndrome. Blood. 2000;95:90-95.
33. Thomas CF Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004;350:2487-2498.
34. Kaneko Y, Suwa A, Ikeda Y, Hirakata M. Pneumocystis jiroveci pneumonia associated with low-dose methotrexate treatment for rheumatoid arthritis: report of two cases and review of the literature. Mod Rheumatol. 2006;16:36-38.
35. Imokawa S, Colby TV, Leslie KO, Helmers RA. Methotrexate pneumonitis: review of the literature and histopathological findings in nine patients. Eur Respir J. 2000;15:373-381.
36. Martin WJ 2nd, Rosenow EC 3rd. Amiodarone pulmonary toxicity. Recognition and pathogenesis (Part 2). Chest. 1988;93:1242-1248.
37. Martin WJ 2nd, Rosenow EC 3rd. Amiodarone pulmonary toxicity. Recognition and pathogenesis (Part I). Chest. 1988;93:1067-1075.
38. Chang SN, Hwang JJ, Hsu KL, et al. Amiodarone-related pneumonitis. J Formos Med Assoc. 2007;106:411-417.
39. Bidad K, Harries-Jones R. Nitrofurantoin lung injury. Age Ageing. 2004;33:414-415.
40. Brutinel WM, Martin WJ 2nd. Chronic nitrofurantoin reaction associated with T-lymphocyte alveolitis. Chest. 1986;89:150-152.
41. Jederlinic PJ, Sicilian L, Gaensler EA. Chronic eosinophilic pneumonia. A report of 19 cases and a review of the literature. Medicine (Baltimore). 1988;67:154-162.
42. Chamberlain DW, Hyland RH, Ross DJ. Diphenylhydantoin-induced lymphocytic interstitial pneumonia. Chest. 1986;90:458-460.
43. Sánchez-Morillas L, Laguna-Martinez JJ, Reaño-Martos M, et al. A case of hypersensitivity syndrome due to phenytoin. J Investig Allergol Clin Immunol. 2008;18:74-75.
44. Zeitz HJ. Bronchial asthma, nasal polyps, and aspirin sensitivity: Samter’s syndrome. Clin Chest Med. 1988;9:567-576.
45. O’Donnell AE, Mappin FG, Sebo TJ, Tazelaar H. Interstitial pneumonitis associated with “crack” cocaine abuse. Chest. 1991;100:1155-1157.
46. Kleerup EC, Koyal SN, Marques-Magallanes JA, et al. Chronic and acute effects of “crack” cocaine on diffusing capacity, membrane diffusion, and pulmonary capillary blood volume in the lung. Chest. 2002;122:629-638.
47. Chau CH, Yew WW, Lee J. Inhaled budesonide in the treatment of talc-induced pulmonary granulomatosis. Respiration. 2003;70:439.
