- Clinical Technology
- Adult Immunization
- Hepatology
- Pediatric Immunization
- Screening
- Psychiatry
- Allergy
- Women's Health
- Cardiology
- Pediatrics
- Dermatology
- Endocrinology
- Pain Management
- Gastroenterology
- Infectious Disease
- Obesity Medicine
- Rheumatology
- Nephrology
- Neurology
- Pulmonology
Respiratory infections in diabetes: Reviewing the risks and challenges
ABSTRACT: Although the organisms that cause community-acquiredpneumonia are similar in diabetic and nondiabetic patients,those who have diabetes mellitus (DM) may have moresevere disease and a poorer prognosis. Elevated blood glucoselevels are associated with worse outcomes in patients withpneumonia, and the mortality risk may be as high as 30% in patientswith uncontrolled DM. Thus, appropriate treatment-and possibly prevention-of bacterial pneumonia should includeaggressive efforts directed at glycemic control. Other respiratoryinfections, such as influenza, tuberculosis, and fungalpneumonia, also are associated with greater morbidity in patientswith DM. Diabetic patients with tuberculosis are morelikely to present with bilateral lung involvement and pleural effusions.(J Respir Dis. 2008;29(7):285-293)
ABSTRACT: Although the organisms that cause community-acquired pneumonia are similar in diabetic and nondiabetic patients, those who have diabetes mellitus (DM) may have more severe disease and a poorer prognosis. Elevated blood glucose levels are associated with worse outcomes in patients with pneumonia, and the mortality risk may be as high as 30% in patients with uncontrolled DM. Thus, appropriate treatment- and possibly prevention-of bacterial pneumonia should include aggressive efforts directed at glycemic control. Other respiratory infections, such as influenza, tuberculosis, and fungal pneumonia, also are associated with greater morbidity in patients with DM. Diabetic patients with tuberculosis are more likely to present with bilateral lung involvement and pleural effusions. (J Respir Dis. 2008;29(7):285-293)
Diabetes mellitus (DM) is a complex metabolic disorder that is characterized by hyperglycemia and is associated with increasing incidence, morbidity, and mortality. According to the World Health Organization (WHO), 180 million persons have DM and 5% of deaths worldwide can be attributed to this disease. Also, there is evidence that DM is associated with an increased risk of infections and with more severe clinical consequences of such infections.1-3
The mechanisms that lead to excess morbidity and mortality are related in part to the host immune defects associated with DM. Coexisting conditions, such as vascular, renal, and cardiovascular diseases, and the various interventions associated with such diseases, contribute significantly to the increased incidence and complexity of infections in patients with DM.
In this article, we will briefly review the immunological and respiratory changes associated with DM. Then we will focus on the challenges of community-acquired pneumonia (CAP) and nosocomial pneumonia in patients with DM.
EFFECTS OF DIABETES
Immunological changes
The airways and alveoli are constantly exposed to microbes, but normal host defense mechanisms can often protect the lungs. In the upper respiratory tract, aerodynamic filtration, mucociliary clearance, cough mechanism, and neurological reflexes prevent aspiration and remove large particles.4 In the lower respiratory tract, defenses include bronchus-associated lymphoid tissue, opsonins (IgA and IgG), surfactant, extracellular chemotactic factors, alveolar macrophages, and pathogen-specific immune responses involving dendritic cells and T and B lymphocytes.5
Overall, the immune response is impaired in persons with DM. Several aspects of cellular immune function-chemotaxis, adherence, phagocytosis, and intracellular killing-are adversely affected by hyperglycemia. Anaerobic conditions in the tissue that are created by vascular compromise and inflammatory response further impair the immune response.
Intracellular killing is altered because hyperglycemia impairs Fc gamma receptor–mediated phagocytosis by neutrophils through inhibition of protein kinase C.6,7 In addition, intracellular killing is impaired because nicotinamide adenine dinucleotide phosphate is consumed by the polyol pathway, thus depleting the amount available for free radical formation.8,9 It has been demonstrated that insulin improves impaired cellular immune functions, both directly and through better glycemic control.10-13
The cell-mediated immune response also is weakened in DM. The diabetic state causes an increase in levels of proinflammatory cytokines, such as tumor necrosis factor α, interleukin (IL)-1B, IL-18, and IL-16. These cytokines promote insulin resistance by decreasing glucose transporter protein 4 receptors and increasing lipolysis. The elevated levels of these cytokines can be reversed by insulin and improved metabolic control.7,8 The humoral immune response, however, appears to be preserved, as evidenced by the adequate response to influenza, pneumococcal, and hepatitis B vaccines.14
Respiratory effects
Pulmonary function may be adversely affected by DM. The mechanical function of the lungs has been evaluated in a number of studies, but the results are contradictory. Some studies have shown reduction in forced vital capacity, forced expiratory volume in 1 second, total lung capacity, and end-expiratory volume, all of which are the result of decreased elastic recoil.15-17 However, other studies have shown that pulmonary function is predominantly preserved in persons with DM.18-20
The pathophysiology of lung abnormalities in patients who have DM is believed to involve microangiopathic changes in the basement membrane of pulmonary blood vessels and respiratory epithelium, as well as nonenzymatic glycosylation of tissue protein.21 Autopsies of patients with DM have revealed thickening of alveolar epithelia, vascular hyalinosis, and pulmonary microangiopathy.22,23 The increased thickness of the alveolar septa leads to diminished diffusion of oxygen.16,18-20,24-26
The extent of the changes in lung tissue seems to be associated with the level of glycemic control, while the changes in pulmonary capillaries mirror the degree of retinopathy, nephropathy, and neuropathy.27 Persons with DM also are susceptible to pulmonary infections because of an increased risk of aspiration secondary to gastroparesis, diminished cough reflex, and disordered sleep patterns.24,28
PULMONARY INFECTIONS
Acute respiratory infections are the number 1 cause of mortality globally with more than 4.5 million deaths a year. In the United States, pneumonia is the sixth most common cause of death and the most prevalent cause of infectious disease–related mortality.29
Chronic underlying conditions, such as immunosuppression, generally increase the risk of pneumonia. However, the evidence of an increased risk of pneumonia in persons with DM is inconsistent. Most research has not shown clearly that pneumonia is more likely to develop in the overall diabetic population. However, the evidence is stronger when subsets of the population are examined. For example, Lipsky and associates30 and Muller and associates1 reported an increased risk of pneumonia among elderly persons with DM. Zanobetti and Schwartz31 identified DM as a risk factor for pneumonia among patients younger than 40 years.
It has also been reported that persons with DM may be more likely to have recurrent pneumonia.14 Some studies have linked diabetes with higher pneumonia-related mortality rates, with one reporting nearly a 30% increase in mortality risk among diabetic persons compared with nondiabetic persons.32,33
Community-acquired pneumonia
Generally, CAP is most prevalent in persons in the sixth and seventh decades of life and occurs predominantly in midwinter and early spring. The rate of hospitalization for patients with CAP has been reported to range from 0% to 50%, with 10% of patients requiring intensive care management.29
In the first population-based, case-control study of DM and pneumonia, researchers reported a 50% increase in the risk of CAP among patients with DM. The risk was especially pronounced among males,younger adults, and patients with no other comorbidities.34 Almirall and associates35 found that on univariate analysis, DM was a risk factor for CAP among adults, but the significance was only marginal and the risk was not significant on bivariate or multivariate analysis.
Another study found that after adjusting for race, sex, and comorbid conditions, there was no significant association between DM and pneumonia due to Streptococcus pneumoniae, the most common cause of CAP.36 Lipsky and colleagues30 reported no increased risk of CAP among persons with DM.
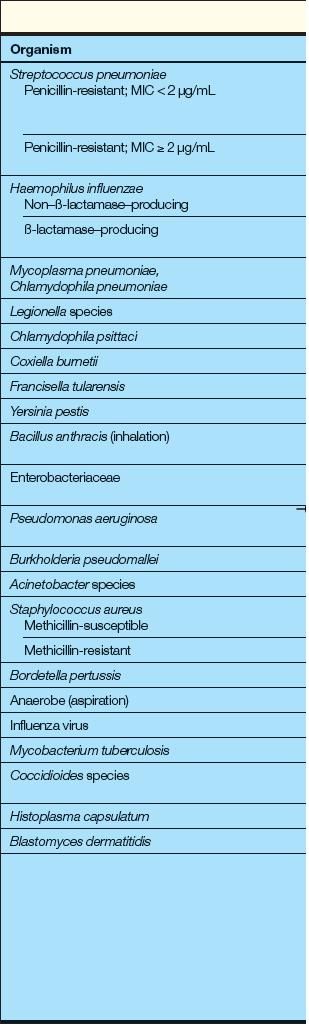
CAP is caused by a variety of bacteria, including atypical pathogens (such as Mycoplasma pneumoniae, Chlamydophila species, and Legionella species), and viruses. The most prevalent organisms among patients with DM are similar to those among the general population and include S pneumoniae and Haemophilus influenzae.8,37
Diabetic patients have increased morbidity from pneumonia caused by Staphylococcus aureus, particularly methicillin-resistant S aureus (MRSA), which is a relatively new pathogen in CAP.8,14,37-39 In fact, they have been found to have increased nasal carriage of S aureus, including MRSA, and insulin use may be associated with increased prevalence of MRSA infections (odds ratio [OR], 3.32; 95% confidence interval [CI], 1.39 - 7.91).14,38,40,41
Although the organisms that cause CAP are similar in diabetic and nondiabetic patients, those with DM may have more severe disease and a poorer prognosis. They tend to be more prone to pleural effusion and multilobar infiltrates.42 Studies also have shown higher rates of bacteremia in diabetic patients with CAP.43,44 Hospitalization appears to be more common among diabetic patients with pneumonia. However, it could be postulated that clinicians simply have a lower threshold for hospitalization when treating those with DM.
Houston and associates45 found no increase in pneumonia morbidity among elderly diabetic patients, and Thomsen and associates46 reported that the death rate from bacteremia appears to be no different in diabetic patients than in the general patient population.
However, a meta-analysis by Fine and colleagues47 indicated a 2% to 30% increase in CAP-related mortality in patients with DM (OR, 1.3; 95% CI, 1.1 - 1.5). In this study, the risk of death from CAP was significantly influenced by smoking, pulmonary disease, and cardiac failure. Ketoacidosis seems to be especially important in the prognosis of diabetic patients with pneumonia.2,48
Nosocomial pneumonia
Nosocomial pneumonia is defined as pneumonia that occurs 48 hours or more after admission and was not incubating at the time of admission. As in CAP, there is controversy surrounding whether diabetic persons are more susceptible to nosocomial respiratory infections. Nosocomial pneumonias in general are more commonly caused by gram-negative bacteria.
It is widely recognized that gram-negative bacilli are important respiratory pathogens in the elderly, especially those with chronic underlying disease.29 Older persons are more likely to be colonized with aerobic gram-negative bacilli. Studies also have found that patients with DM are more frequently colonized with gram-negative organisms29 and that the incidence of infections caused by these organisms is higher in these patients.14,38,48
Mackowiak and colleagues49 examined the likelihood of gram-negative colonization in aspiration-prone patients, including those with DM, alcoholism, narcotic addiction, and seizure disorders. They found that the risk of colonization with Enterobacter species, Klebsiella pneumoniae, and Escherichia coli was increased by 24% and 14% in patients with DM and alcoholism, respectively, compared with controls. DM and alcoholism were found to be the most common underlying diseases in patients who had already had gram-negative bacillary pneumonia.
Another study found that about half of the study population of non–insulin-dependent diabetic patients were colonized with gram-negative species, and the colonization rates increased with older age and longer duration of disease.50 However, Vardakas and associates51 reported that nosocomial pneumonia was not more likely to develop in diabetic patients in the ICU than in other patients. Akbar37 reported that the mortality rates for diabetic and nondiabetic patients with nosocomial pneumonia did not differ.
A typical bacterial pneumonia
A typical organisms, such as Chlamydophila pneumoniae and M pneumoniae, have had no reported association with DM.8 However, an increased prevalence of Legionella pneumonia has been linked with DM. In a review of community acquired legionellosis, the risk of acquiring Legionella pneumonia was increased in diabetic patients.52
Diagnosis and management of bacterial pneumonia

The symptoms suggestive of pneumonia are cough, shortness of breath, and pleuritic chest pain. Physical examination findings may include fever, tachypnea, tachycardia, and audible rales on lung examination. A demonstrable infiltrate on a chest radiograph with or without supporting microbiological data is required for the diagnosis of pneumonia.
Sputum Gram stain and cultures can be helpful in choosing the initial antibiotic regimen and in narrowing, broadening, or switching the antibiotic regimen. Additional diagnostic procedures may include blood cultures, testing for Legionella urinary antigen and pneumococcal urinary antigen, endotracheal aspiration (if the patient is intubated), and possibly bronchoscopic or nonbronchoscopic bronchoalveolar lavage (BAL) in patients who require ICU admission. Fungal and mycobacterial cultures are indicated if the patient has cavitary infiltrates. Thoracentesis and pleural fluid cultures are indicated if pleural effusion is detected.53
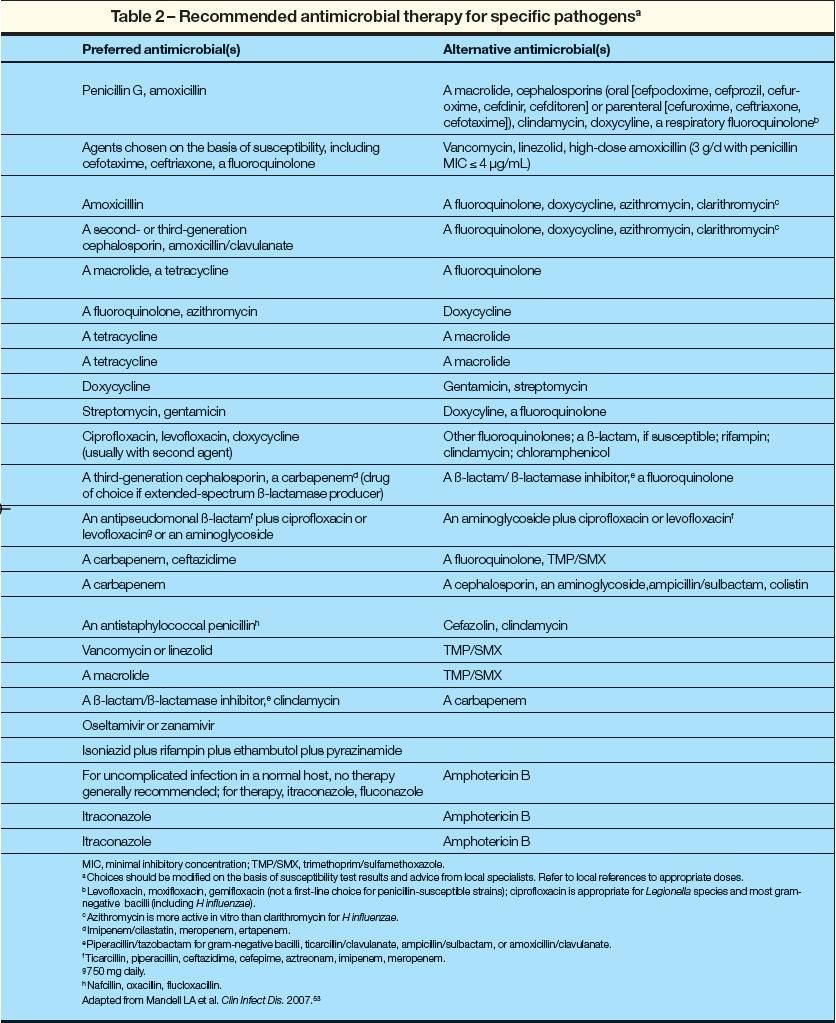
The same antibiotic regimens are used in diabetic patients as in the general population (Table 1). Treatment options may be simplified if the causal agent is established or strongly suspected (Table 2a and 2b).
Blood glucose control is another aspect of therapy for pneumonia in diabetic patients. Although most studies evaluating the incidence and prognosis of bacterial pneumonia fail to include data on glycemic control, it is widely recognized that elevated blood glucose levels negatively affect outcomes in diabetic patients with pneumonia, with a mortality risk as high as 30% in cases of uncontrolled DM.33,54-56
Fine and associates57 developed a prediction rule to stratify patients with CAP according to the risk of death. In this model, blood glucose levels higher than 200 mg/dL are associated with a 10-point increase in risk, with 130 points being extreme high risk. A study evaluating intubated patients discovered that those with glucose in their airway secretions were more likely to have growth of pathogenic bacteria, including MRSA, and infiltrates on chest radiographs.39 Thus, appropriate treatment-and possibly prevention-of bacterial pneumonia in diabetic patients should include aggressive efforts directed at glycemic control.
Other novel treatment regimens may include statins. Van de Garde and colleagues58 found that statin use helped prevent respiratory infections in persons with DM. The exact mechanism is still speculative, but there is evidence that statins have immunomodulatory and anti-inflammatory properties.
Furthermore, diabetic patients respond well to pneumococcal and influenza vaccinations, making timely and appropriate immunization a cost-effective and efficient method of preventing morbidity from pneumococcal and influenza pneumonia in these patients.38,48
Pulmonary tuberculosis
Mycobacterium tuberculosis infects about a third of the world's population and is responsible for about 2 million deaths per year. Normally, M tuberculosis is only a moderately infectious agent, but risk factors for progression from infection (latent tuberculosis) to disease include an impaired immune system, increased duration and intensity of pathogen exposure, malnutrition, alcohol abuse, homelessness, incarceration, host genetic factors, and-most significantly-AIDS. Patients at risk for increased morbidity from tuberculosis include injection drug users, residents of institutions such as homeless shelters and nursing homes, and those with end-stage renal disease.59
On the basis of Richard Morton's 1694 Phthisiologia: Or, a Treatise of Consumptions, the association between DM and tuberculosis has been recognized since the Roman era.60,61 Autopsies performed on diabetic persons before 1900 revealed that about half of them had tuberculosis, and surveys before 1960 showed that tuberculosis was 2 to 4 times more prevalent in diabetic persons.
After the introduction of insulin, oral hypoglycemics, and improved antibiotics, the significance of DM and tuberculosis as comorbid conditions waned until the recent worldwide burgeoning of both diseases. In fact, 8 of the 10 countries with the highest prevalence of DM are labeled by the WHO as "high burden" states for tuberculosis.62
Several studies have shown that either the prevalence of tuberculosis is increased in diabetic patients or the propensity for hyperglycemia and DM is greater in persons with tuberculosis.63-66 These studies included both new and reactivated tuberculosis cases, and most of the research was conducted among Asian Indian, African, and Hispanic populations. The reported risk of tuberculosis in diabetic patients ranged from 4% to 14%.63-66
However, most of these studies were retrospective, did not control for other immunocompromised states (such as HIV infection), and failed to report glycemic control. In addition, the researchers did not address the fact that diabetic patients receive more medical treatment and tuberculosis screening than the nondiabetic population.62
The presentation of tuberculosis (fever, cough, and weight loss) is similar in diabetic and nondiabetic patients, but those with DM are more likely to present with bilateral lung involvement and pleural effusions. Numerous researchers have also reported an increased prevalence of cavitary lung lesions and lower lobe involvement.63,67-69 Mortality from tuberculosis has been reported to be approximately 10% among diabetic patients, and hospitalization rates are higher in those who are older than 65 years than in nondiabetic patients in the same age group.63
While some studies have found no evidence of an increased prevalence of multidrug-resistant tuberculosis among diabetic patients, at least 1 study reported an increased risk, with nearly 40% of the diabetic patients being infected with a resistant strain.70-72
Common drugs that are used for treatment of tuberculosis are rifampin, isoniazid, pyrazinamide, and ethambutol. The treatment for tuberculosis in patients with DM is similar to that in nondiabetic patients. However, one study demonstrated that serum rifampin levels are at least 2 times higher in nondiabetic controls than in diabetic patients.70 In addition, higher dosesof oral hypoglycemics are needed because of interaction with rifampin. Pyridoxine supplementation is recommended to prevent nerve damage by isoniazid.48
Viral pneumonia
Both respiratory syncytial virus and influenza A virus are significant causes of pneumonia. Bacterial coinfection occurs in almost 20% of cases and often makes the cause of the pneumonia unclear.29
Peleg and associates14 demonstrated that diabetic patients have greater morbidity from influenza. Specifically, they found that these patients had 6 times more hospitalizations during an influenza epidemic than persons in the general population. The authors postulated that the increased rate of hospitalization might be the result of coinfection with S aureus, multiple comorbidities, and metabolic compromise.
Koziel and Koziel8 attributed the increased hospitalization rate among diabetic patients with pneumonia to the presence of ketoacidosis. Bouter and colleagues44 reported a 50% increase in the number of cases of ketoacidosis during influenza epidemics. Furthermore, the risk of pneumonia-related mortality among diabetic persons has been shown to be increased during influenza epidemics.44,73
The diagnosis can be made by cultures, serology, enzyme immunoassay, immunofluorescence, and polymerase chain reaction.
Fungal pneumonia
There is a widespread assumption that the incidence of fungal infections is increased in diabetic patients and that the clinical course is more severe, but there is little evidence to support this. There are reports that DM may predispose patients to endemic dimorphic fungal infections. No study has provided statistical evidence that the risk of an endemic mycosis is greater in persons with well-controlled DM than in nondiabetic persons; however, the risk of systemic infection with an endemic fungus may be increased in persons with poorly controlled diabetes.
Several fungi, including Mucor species, Aspergillus species, Coccidioides immitis, and Cryptococcus neoformans, can cause primary pneumonia. Radiographic finding may include subacute, chronic infiltrates that can evolve into lung abscesses and rapidly progressive pneumonia.74
Aspergillosis may cause acute or chronic pneumonia, presenting as an intracavitary mass or mycetoma. Chronic Aspergillus pneumonia is associated with nonspecific symptoms, such as fever and productive cough. Radiographically, it may present as infiltrates and cavitary disease, which may appear to be more like tuberculosis or anaerobic lung abscess. Patients may have symptoms for months before the diagnosis is made. BAL, transthoracic percutaneous needle aspiration, and video-assisted thoracoscopic biopsy are standard procedures for establishing the diagnosis of pulmonary aspergillosis.
Because of better survival and improved responses to initial therapy with voriconazole, primary therapy with amphotericin B is not recommended. For primary treatment of invasive pulmonary aspergillosis, intravenous or oral voriconazole is indicated for most patients. Oral therapy can be maximized by using a dosage of 4 mg/kg (rounded up to pill sizes). For seriously ill patients, the parenteral formulation is recommended.
For salvage therapy, antifungal agents include liposomal amphotericin B, posaconazole, itraconazole, and caspofungin. Micafungin and anidulafungin have activity against Aspergillus species but are not yet approved for this use. Use of an azole should take into account previous therapy, host factors, and pharmacokinetic considerations. Because long-term treatment is required, oral antifungal therapy is preferred over parenteral therapy.75
Coccidioidomycosis and cryptococcal pneumonia rarely occur in diabetic patients. In a case-control study, DM was identified as an independent risk factor for severe pulmonary coccidioidomycosis.76 In a retrospective review of coccidioidomycosis in patients with DM, Santelli and associates77 found that patients with DM were more likely to have cavitary lung infection, were more likely to experience relapse, were less likely to have resolution, and had an increased risk of disseminated coccidioidomycosis.
Although patients with DM may have a high carrier rate for Candida, pneumonia is not a common complication. The diagnosis of primary Candida pneumonia is rare and requires histopathological confirmation. Secondary pneumonia is more likely to occur in severely immunocompromised patients who have hematogenously disseminated candidiasis.
Therapy is directed at disseminated candidiasis. Antifungal agents that have activity against the most common species, such as Candida albicans, are the polyenes (amphotericin B, including the lipid and liposomal formulations), azoles (fluconazole), and echinocandins (caspofungin, micafungin, and anidulafungin). Most patients with primary Candida pneumonia have been treated with amphotericin B (0.7 to 1.0 mg/kg/d). Fluconazole can be used in milder cases.
Candida glabrata has reduced susceptibility to azoles and amphotericin B; echinocandins are good alternatives, but many authorities still prefer amphotericin B. Candida lusitaniae is resistant to amphotericin B, and fluconazole is the preferred therapy for infections caused by this species. Voriconazole is a broad spectrum azole that is active against Candida species. Other azoles, such as itraconazole and posaconazole, are not approved for hematogenously disseminated candidiasis.78
The treatment of other fungal infections and endemic mycoses depends on the specific pathogen.
References:
REFERENCES
1.
Muller LM, Gorter KJ, Hak E, et al. Increased risk of common infections in patients with type 1 and 2 diabetes mellitus.
Clin Infect Dis.
2005;41:281-288.
2.
Kaslow RA. Infections in diabetes. In: Harris MI, Hammon RF, eds.
Diabetes in America.
Bethesda, MD: National Institutes of Health; 1985:XIXI-18. NIH publication 85-1468.
3.
Infections and diabetes mellitus.
West J Med.
1979;130:515-521.
4.
Reynolds HY. Integrated host defense against infections. In: Crystal RG, West JB, eds.
The Lung: Scientific Foundations.
New York: Raven Press Ltd; 1991:1899.
5.
Shelhamer JH, Toews GB, Masur H, et al. NIH conference. Respiratory disease in the immunosuppressed patient.
Ann Intern Med.
1992;117:415-431.
6.
Saiepour D, Sehlin J, Oldenborg PA. Insulin inhibits phagocytosis in normal human neutrophils via PKC alpha/beta-dependent priming of F-actin assembly.
Inflamm Res.
2006;55:85-91.
7.
Turina M, Fry DE, Polk HC Jr. Acute hyperglycemia and innate immune system: clinical, cellular, and molecular aspects.
Crit Care Med.
2005;33:1624-1633.
8.
Koziel H, Koziel MJ. Pulmonary complications of diabetes mellitus. Infect Dis Clin North Am. 1995;9:65-96.
9.
Bhattacharya SK, Shastri S, Mahajan P, et al. Polymorphonuclear leukocyte function in type-2 diabetes mellitus patients and its correlation with glycemic control.
Nepal Med Coll J.
2007;9:111-116.
10.
Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus (DM).
FEMS Immunol Med Microbiol.
1999;26:259-265.
11.
Delamaire M, Maugendre D, Moreno M, et al. Impaired leukocyte function in diabetic patients.
Diabet Med.
1977;14:29-34.
12.
Valerius NH, Eff C, Hansen NE, et al. Neutrophil and lymphocyte function in patients with diabetes mellitus.
Acta Med Scand.
1982;211:463-472.
13.
Ljubic S, Metelko Z, Car N, et al. Reduction of diffusion capacity for carbon monoxide in diabetic patients.
Chest.
1998;114:1033-1035.
14.
Peleg AY, Weerarathna T, McCarthy JS, Davis TM. Common infections in diabetes: pathogenesis, management and relationship to glycaemic control.
Diabetes Metab Res Rev.
2007;23:3-13.
15.
Lange P, Parner J, Schnohr P, Jensen G. Copenhagen City Heart Study: longitudinal analysis of ventilatory capacity in diabetic and nondiabetic adults.
Eur Respir J.
2002;20:1406-1412.
16.
Schnack C, Festa A, Schwarzmaier-D'Assié A, et al. Pulmonary dysfunction in type 1 diabetes in relation to metabolic long-term control and to incipient diabetic nephropathy.
Nephron.
1996;74:395-400.
17.
Davis TM, Knuiman M, Kendall P, et al. Reduced pulmonary function and its associations in type 2 diabetes: the Fremantle Diabetes study.
Diabetes Res Clin Pract.
2000;50:153-159.
18.
Strojek K, Ziora D, Sroczynski JW, Oklek K. Pulmonary complications of type 1 (insulin-dependent) diabetic patients.
Diabetologia.
1992;35:1173-1176.
19.
Fuso L, Cotroneo P, Basso S, et al. Postural variations of pulmonary diffusing capacity in insulindependent diabetes mellitus.
Chest.
1996;110:1009-1013.
20.
Benbassat CA, Stern E, Kramer M, et al. Pulmonary function in patients with diabetes mellitus.
Am J Med Sci.
2001;322:127-132.
21.
Sandler M, Bunn AE, Stewart RI. Cross-section study of pulmonary function in patients with insulindependent diabetes mellitus.
Am Rev Respir Dis.
1987;135:223-229.
22.
Vracko R, Thorning D, Huang TW. Basal lamina of alveolar epithelium and capillaries: quantitive changes with aging and diabetes mellitus.
Am Rev Respir Dis.
1979;120:973-983.
23.
Kodolova IM, Lysenko LV, Saltykov BB. Changes in the lungs in diabetes mellitus [in Russian].
Arkh Patol.
1982;44:35-40.
24.
Mowat A, Baum J. Chemotaxis of polymorphonuclear leukocytes from patients with diabetesmellitus.
N Engl J Med.
1971;284:621-627.
25.
Ramirez LC, Dal Nogare A, Hsia C, et al. Relationship between diabetes control and pulmonary infection in insulin-dependent diabetes mellitus.
Am J Med.
1991;91:371-376.
26.
Niranjan V, McBrayer DG, Ramirez LC, et al. Glycemic control and cardiopulmonary function in patients with insulin-dependent diabetes mellitus.
Am J Med.
1997;103:504-513.
27.
Hsia CC, Raskin P. The diabetic lung: relevance of alveolar microangiopathy for the use of inhaled insulin.
Am J Med.
2005;118:205-211.
28.
Antonelli Incalzi R, Fuso L, Giordano A, et al. Neuroadrenergic denervation of the lung in type 1 diabetes mellitus complicated by autonomic neuropathy.
Chest.
2002;121:443-451.
29.
Donowitz GR, Mandell GL. Acute pneumonia. In: Mandell GL, Bennett JE, Dolin R, eds.
Principles and Practice of Infectious Diseases.
6th ed. Philadelphia: Churchill and Livingstone; 2005.
30.
Lipsky BA, Boyko EJ, Inui TS, Koepsell TD. Risk factors for acquiring pneumococcal infections.
Arch Intern Med.
1986;146:2179-2185.
31.
Zanobetti A, Schwartz J. Are diabetics more susceptible to the health effects of airborne particles.
Am J Respir Crit Care Med.
2001;164:831-833.
32.
Sarnak MJ, Jaber BL. Pulmonary infectious mortality among patients with end-stage renal disease.
Chest.
2001;120:1883-1887.
33.
Kornum JB, Thomsen RW, Riis A, et al. Type 2 diabetes and pneumonia outcomes: a populationbased cohort study.
Diabetes Care.
2007;30:2251-2257.
34.
Thomsen RW, Hundborg HH, Lervang HH, et al. Risk of community-acquired pneumococcal bacteremia in patients with diabetes: a populationbased case-control study.
Diabetes Care.
2004;27:1143-1147.
35.
Almirall J, BolÃbar I, Balanzó X, González CA. Risk factors for community-acquired pneumonia in adults: a population-based case-control study.
Eur Respir J.
1999;13:349-355.
36.
Nuorti JB, Butler JC, Farley MM, et al. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team.
N Engl J Med.
2000;342:681-689.
37.
Akbar DH. Bacterial pneumonia: comparison between diabetics and non-diabetics.
Acta Diabetol.
2001;38:77-82.
38.
Joshi N, Caputo GM, Wietekamp MR, Karchmer AW. Infections in patients with diabetes mellitus.
N Engl J Med.
1999;341:1906-1912.
39.
Philips BJ, Redman J, Brennan A, et al. Glucose in bronchial aspirates increases the risk of respiratory MRSA in intubated patients.
Thorax.
2005;60:761-764.
40.
Graham PL III, Lin SX, Larson EL. A U.S. population-based survey of Staphylococcus aureus colonization.
Ann Intern Med.
2006;144:318-325.
41.
Tamer A, Karabay O, Ekerbicer H. Staphylococcus aureus nasal carriage and associated factors in type 2 diabetic patients.
Jpn J Infect Dis.
2006;59:10-14.
42.
Falguera M, Pifarre R, Martin A, et al. Etiology and outcome of community-acquired pneumonia in patients with diabetes mellitus.
Chest.
2005;128:3233-3239.
43.
Marrie TJ. Bacteraemic pneumococcal pneumonia: a continuously evolving disease.
J Infect.
1992;24:247-255.
44.
Bouter KP, Diepersloot RJ, van Romunde LK, et al. Effect of epidemic influenza on ketoacidosis, pneumonia and death in diabetes mellitus: a hospital register survey of 1976-1979 in The Netherlands.
Diabetes Res Clin Pract.
1991;12:61-68.
45.
Houston MS, Silverstein MD, Suman VJ. Risk factors for 30-day mortality in elderly patients with lower respiratory tract infection. Community-based study.
Arch Intern Med.
1997;157:2190-2195.
46.
Thomsen RW, Hundborg HH, Lervang HH, et al. Diabetes and outcomes of community-acquired pneumococcal bacteremia: a 10-year populationbased cohort study.
Diabetes Care.
2004;27:70-76.
47.
Fine MJ, Smith MA, Carson CA, et al. Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis.
JAMA.
1996;275:134-141.
48.
Gupta S, Koirala J, Khardori R, Khardori N. Infections in diabetes mellitus and hyperglycemia.
Infect Dis Clin North Am.
2007;21:617-638.
49.
Mackowiak PA, Martin RM, Jones SR, Smith JW. Pharyngeal colonization by gram-negative bacilli in aspiration-prone persons.
Arch Intern Med.
1978;138:1224-1227.
50.
Aswapokee N, Pruksachatvuthi S, Rungpitarungsi V, Vichayanrat A. Pharyngeal colonization by gram-negative bacilli in ambulatory diabetics.
J Med Assoc Thai.
1985;68:243-247.
51.
Vardakas KZ, Siempos II, Falagas ME. Diabetes mellitus as a risk factor for nosocomial pneumonia and associated mortality.
Diabet Med.
2007;24:1168-1171.
52.
Den Boer JW, Nijhof J, Friesema I. Risk factors for sporadic community-acquired Legionnaires' disease. A 3-year national case-control study.
Public Health.
2006;120:566-571.
53.
Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults.
Clin Infect Dis.
2007;44(suppl 2):S27-S72.
54.
McAlister FA, Majumdar SR, Blitz S, et al. The relationship between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community-acquired pneumonia.
Diabetes Care.
2005;28:810-815.
55.
Krinsley JS. Effect of intensive glucose management protocol on the mortality of critically ill adult patients [published correction appears in
Mayo Clin Proc.
2004;80:1101].
Mayo Clin Proc.
2004;79:992-1000.
56.
van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients.
N Engl J Med.
2001;345:1359-1367.
57.
Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with communityacquired pneumonia.
N Engl J Med.
1997;336:243-250.
58.
van de Garde EM, Hak E, Souverein PC, et al. Statin treatment and reduced risk of pneumonia in patients with diabetes.
Thorax.
2006;61:957-961.
59.
Fitzgerald D, Haas DW. Mycobacterium tuberculosis. In: Mandell GL, Bennett JE, Dolin R, eds.
Principles and Practice of Infectious Diseases.
6th ed. Philadelphia: Churchill Livingstone; 2005.
60.
Dixon B. Diabetes and tuberculosis: an unhealthy partnership.
Lancet Infect Dis.
2007;7:444.
61.
Broxmeyer L. Diabetes mellitus, tuberculosis and the mycobacteria: two millenia of enigma.
Med Hypotheses.
2005;65:433-439.
62.
Restrepo BI. Convergence of the tuberculosis and diabetes epidemics: renewal of old acquaintances.
Clin Infect Dis.
2007;45:436-438.
63.
Jabbar A, Hussain SF, Khan AA. Clinical characteristics of pulmonary tuberculosis in adult Pakistani patients with co-existing diabetes mellitus.
East Mediterr Health J.
2006;12:522-527.
64.
Ponce-De-Leon A, Garcia-Garcia Md Mde L, Garcia-Sancho MC, et al. Tuberculosis and diabetes in southern Mexico.
Diabetes Care.
2004;27:1584-1590.
65.
Stevenson CR, Forouhi NG, Roglic G, et al. Diabetes and tuberculosis: the impact of the diabetes epidemic on tuberculosis incidence.
BMC Public Health.
2007;7:234.
66.
Pablos-Méndez A, Blustein J, Knirsch CA. The role of diabetes mellitus in the higher prevalence of tuberculosis among Hispanics.
Am J Public Health.
1999;87:574-579.
67.
Feleke Y, Abdulkadir J, Aderaye G. Prevalence and clinical features of tuberculosis in Ethiopian diabetic patients.
East Afr Med J.
1999;76:361-364.
68.
Pérez-Guzman C, Torres-Cruz A, Villarreal-Velarde H, et al. Progressive age-related changes in pulmonary tuberculosis images and the effect of diabetes.
Am J Respir Crit Care Med.
2000;162:1738-1740.
69.
Pérez-Guzman C, Torres-Cruz A, Villareal-Velarde H, et al. Atypical radiological images of pulmonary tuberculosis in 192 diabetic patients: a comparative study.
Int J Tuberc Lung Dis.
2001;5:455-461.
70.
Alisjahbana B, Sahiratmadja E, Nelwan EJ, et al. The effect of type 2 diabetes mellitus on the presentation and treatment response of pulmonary tuberculosis.
Clin Infect Dis.
2007;45:428-435.
71.
Singla R, Khan N. Does diabetes predispose to the development of multidrug-resistant tuberculosis?
Chest.
2003;123:308-309.
72.
Bashar M, Alcabes P, Rom WN, Condos R. Increased incidence of multidrug-resistant tuberculosis in diabetic patients on the Bellevue Chest Service, 1987 to 1997.
Chest.
2001;120:1514-1519.
73.
Barker WH, Mullooly JP. Pneumonia and influenza deaths during epidemics: implications for prevention.
Arch Intern Med.
1982;142:85-89.
74.
Rubin SA, Chaljub G, Winer-Muram HT, Flicker S. Pulmonary zygomycosis: a radiographic and clinical spectrum.
J Thorac Imaging.
1992;7:85-90.
75.
Walsh TJ, Anaissie EJ, Denning DW, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America.
Clin Infect Dis.
2008;46:327-360.
76.
Rosenstein NE, Emery KW, Werner SB, et al. Risk factors for severe pulmonary and disseminated coccidioidomycosis: Kern County, California, 1995-1996.
Clin Infect Dis.
2001;32:708-715.
77.
Santelli AC, Blair JE, Roust LR. Coccidioidomycosis in patients with diabetes mellitus.
Am J Med.
2006;119:964-969.
78.
Pappas PG, Rex JH, Sobel JD, et al. Guidelines for treatment of candidiasis.
Clin Infect Dis.
2004;38:161-189.
