- Clinical Technology
- Adult Immunization
- Hepatology
- Pediatric Immunization
- Screening
- Psychiatry
- Allergy
- Women's Health
- Cardiology
- Pediatrics
- Dermatology
- Endocrinology
- Pain Management
- Gastroenterology
- Infectious Disease
- Obesity Medicine
- Rheumatology
- Nephrology
- Neurology
- Pulmonology
Inhalational anthrax, part 2: Prevention and treatment
When untreated, inhalational anthrax typically resultsin a rapidly fatal illness. Evidence suggests that both theanthrax vaccine and prophylaxis with ciprofloxacin or doxycyclineare effective in preventing illness after inhalational anthraxexposure. The current anthrax vaccine appears to have anadverse-effect profile that is similar to that of other adult vaccines.For patients with active infection, the CDC recommendsa multi-antibiotic regimen that should include doxycycline ora fluoroquinolone and 2 additional antibiotics that have proteinor RNA synthesis inhibition, such as rifampin and clindamycin.Monoclonal antibodies directed against anthrax toxinsmay also play a role in treating active infection. (J Respir Dis.2008;29(6):249-254)
ABSTRACT: When untreated, inhalational anthrax typically results in a rapidly fatal illness. Evidence suggests that both the anthrax vaccine and prophylaxis with ciprofloxacin or doxycycline are effective in preventing illness after inhalational anthrax exposure. The current anthrax vaccine appears to have an adverse-effect profile that is similar to that of other adult vaccines. For patients with active infection, the CDC recommends a multi-antibiotic regimen that should include doxycycline or a fluoroquinolone and 2 additional antibiotics that have protein or RNA synthesis inhibition, such as rifampin and clindamycin. Monoclonal antibodies directed against anthrax toxins may also play a role in treating active infection. (J Respir Dis. 2008;29(6):249-254)
Early detection and rapid initiation of multidrug therapy during the 2001 US anthrax attack probably reduced mortality compared with historical reports. During a future bioterrorist attack, failure to detect anthrax in a timely manner will delay lifesaving therapy, resulting in increased anthrax-associated mortality. Because symptomatic patients will first present to primary care providers and to other outpatient medical settings, it is imperative that all clinicians have an awareness of the clinical signs andsymptoms of inhalational anthrax.
In the May 2008 issue of The Journal of Respiratory Diseases, we reviewed the clinical course of inhalational anthrax and the diagnostic evaluation. In this article, we will address the strategies for prophylaxis and management.
TREATMENT
Preexposure vaccination
In the 1950s, the US army and the CDC developed and tested an acellular human anthrax vaccine (aluminum hydroxide–absorbed protective-antigen vaccine) in placebocontrolled trials involving workers with high-risk exposure to anthrax particles.1,2 These studies showed a 93% efficacy in preventing cutaneous infection. The vaccine is thought to be effective against inhalational anthrax because there have been no known cases of inhalational anthrax in immunized workers with high-risk occupations. 1 However, given the rarity of occupational inhalational anthrax, it is impossible to draw statistical conclusions from these experiments.3
Animal data suggest that the current US-licensed vaccine is highly effective in preventing anthrax infection after inhalational exposure.4,5 In humans, this vaccine induces an immune response in 83% after the first dose, 91% to 95% after the second dose, and 100% after the third dose.6-9 After 2 doses, 95% to 100% of vaccinees have detectable anti–protective antigen (PA) IgG antibodies.9 (Anti-PA antibodies developed in all of the survivors of inhalational anthrax during the 2001 attack.10)
The duration of efficacy is unknown, but animal studies suggest that the vaccine is effective at least 2 years after the administration of 2 doses.5,11,12 The CDC's Advisory Committee on Immunization Practices (ACIP) recommends that only civilian groups that are at high risk for repeated anthrax exposure (such as laboratory workers handling anthrax and high-risk veterinarian workers) be given preexposure vaccination.13
Concerns regarding the safety of the current US-licensed anthrax vaccine have been raised,14,15 but these concerns are not convincingly supported by the literature. Between January 1990 and August 2000, more than 1.8 million doses of anthrax vaccine were administered in the United States, mostly to military personnel.16 Aside from injection site reactions, no serious adverse events were clearly associated with the anthrax vaccine.
Furthermore, no serious adverse events attributed to anthrax vaccination occurred in 1700 patients who were vaccinated and studied during the 2001 attack.17 A National Academy of Sciences expert committee concluded that the current US-licensed anthrax vaccine has an adverse-effect profile that is similar to that of other adult vaccines.18
Prophylaxis
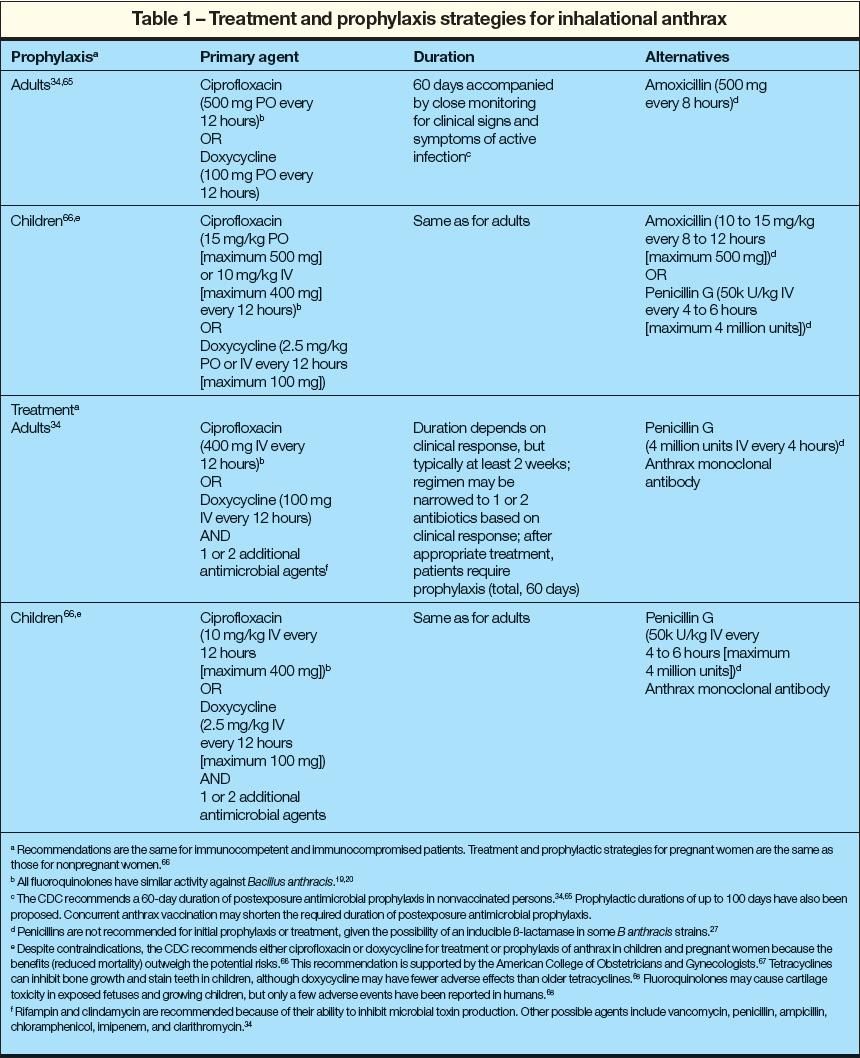
Animal studies suggest that ciprofloxacin and doxycycline are equally effective in preventing symptomatic infection after inhalational exposure.19,20 Other studies show ciprofloxacin, gatifloxacin, and moxifloxacin have similar prophylactic efficacy.21 There is increasing evidence that antibiotic prophylaxis for persons exposed to anthrax particles during the 2001 attack prevented additional active infections22-25 despite adherence rates of only about 50% in potentially exposed persons.22 Efforts to increase antimicrobial adherence are highly cost-effective because of the effectiveness of prophylaxis and the potentially fatal course of symptomatic disease.26
Because of the potential for anthrax to sporulate up to 60 days after exposure, the CDC recommends at least 60 days of antibiotic prophylaxis.27 However, longer prophylactic durations may be warranted for persons exposed to moderate to high doses of anthrax particles.28 Given the animal data of sporulationup to 100 days postexposure,29 longer durations and other optional prophylactic strategies have been suggested.27
Animal data suggest that the duration of antibiotic prophylaxis may be reduced (less than 60 days) if concurrent anthrax vaccination is given.30 Protection against anthrax particle rechallenge is provided by combining postexposure antibiotic treatment with vaccination.19 The CDC's ACIP recommends that prophylaxis may be discontinued 7 to 14 days after the administration of 3 doses of anthrax vaccine (0, 2, and 4 weeks).13 Antimicrobial prophylaxis in combination with anthrax vaccination is highly cost-effective.31,32
Treating active infection
The reduced mortality rate observed during the 2001 attack (46%) compared with historical reports of inhalational anthrax (92%) has been attributed, in part, to rapid diagnosis, multidrug regimens, and timely provision of appropriate therapy during the prodromal phase.33 The CDC recommends a multi-antibiotic regimen to treat active inhalational anthrax (Table 1).34 This regimen should include doxycycline or a fluoro
quinolone (such as ciprofloxacin) and 2 additional antibiotics (antibiotics with protein or RNA synthesis inhibition, such as rifampin and clindamycin, are preferred). In addition to antibiotic therapy, drainage of pleural effusions may be necessary. Most survivors of inhalational anthrax (83%) required repeated drainage for recurrent pleural effusions.33
Before the advent of antibiotics, anthrax antiserum was used to treat and cure patients with inhalational anthrax.33 Antiserum therapy decreased mortality by 75%.35 Because anthrax virulence is primarily due to the production of bacterial toxins,36 antiserum and other therapies directed against these toxins may be superior to antibiotic therapy.37-42 This approach is supported by animal models in which neutralizing monoclonal antibodies showed superior efficacy.11,43,44
Given this evidence, monoclonal antibodies directed against anthrax toxins have been manufactured and stockpiled by both the CDC (for the Strategic National Stockpile) and the US military.45 In a recent report, a person with inhalational anthrax survived after receiving a multidrug antibiotic regimen and monoclonal antibody therapy, even though the disease progressed to the fulminant phase and the person required mechanical ventilation.46 Previously, inhalational anthrax was almost always fatal (97%) in patients whose disease progressed to the fulminant phase (despite antibiotics), and no patient had survived after requiring mechanical ventilation.33
BIOTERRORISM PREPAREDNESS EFFORTS
Given that untreated inhalational anthrax is rapidly fatal, it is imperative that clinicians can accurately identify the characteristic clinical features that distinguish patients with inhalational anthrax from those with respiratory symptoms from other causes.33 Several algorithms have been developed to help health care providers differentiate inhalational anthrax from influenza- like illness or pneumonia.47-54 Unfortunately, these algorithms have several potential limitations.55
Although these algorithms were developed by comparing persons exposed to inhalational anthrax with patients who have flu-like illness or pneumonia, none incorporated symptomatic patients without infectious illness (such as those with cough from asthma or gastroesophageal reflux disease, sinus complaints from allergies, neurological complaints from stroke, or migraines). Because the symptoms of prodromal inhalational anthrax are relatively common in the general population (daily prevalence of up to 10%),56 these algorithms have poor specificity when applied to broad patient populations.
Because knowledge of potential exposure influences patient behavior, during a future bioterrorist attack, many patients with respiratory symptoms not caused by inhalational anthrax will likely inundate emergency departments (EDs) and other health care settings, further limiting the effectiveness of these algorithms. Finally, none of these algorithms have been prospectively validated, so their true diagnostic accuracy is unknown.
In an effort to improve early detection during a bioterrorist attack, the CDC and other public health agencies have developed syndromic surveillance systems.57,58 These systems track ED and outpatient clinic visits, laboratory orders, and pharmaceutical prescriptions and assess for trends to detect disease outbreaks more rapidly. However, these syndromic surveillance methods may have little efficacy beyond that of the standard detection methods (such as the training of "astute clinicians").59,60 For example, a recent study found that during a simulated large-scale anthrax attack, when sensitivity to syndromic surveillance was maintained at 100%, frequent false alarms (as many as 1 every 10 days) occurred, resulting in a modest (0.3- to 1-day) improvement in detection time over standard methods.59
Furthermore, syndromic surveillance systems are costly to institute and maintain.59 The high false alarm rates can take up scarce public health resources and can cause "alarm fatigue" in public health personnel.
In the event of a bioterrorist attack, 2 primary inventories of pharmaceutical and medical supplies will be used: local inventories and the Strategic National Stockpile-a national inventory of antibiotics, chemical antidotes, and ventilators developed to supplement local inventories.61,62 It is recommended that hospitals and other local public health entities have at least a 24- to 48-hour supply of antibiotics to treat and provide prophylaxis for first responders and persons potentially exposed to bioterrorist agents.63
Despite these recommendations, there is no clear consensus about what local inventories should contain. In addition, there is little published information on the quantities and types of medical and pharmaceutical supplies in local inventories.26 One study of 10 hospitals in New Jersey (serving an estimated 1.6 million persons) found a total of289 doses of ciprofloxacin and 175 doses of doxycycline on hand per hospital.64
During a large-scale anthrax attack, tens of thousands of potentially exposed persons will require prophylaxis and treatment. One analysis suggests that mortality from a large-scale inhalational anthrax attack is highly dependent on the number of persons requiring and adhering to prophylactic therapy.26 Improving the dispensing capacity of prophylactic antibiotics at the local level reduces the anticipated mortality more than stockpiling and maintaining local antibiotic inventories.26
CONCLUSION
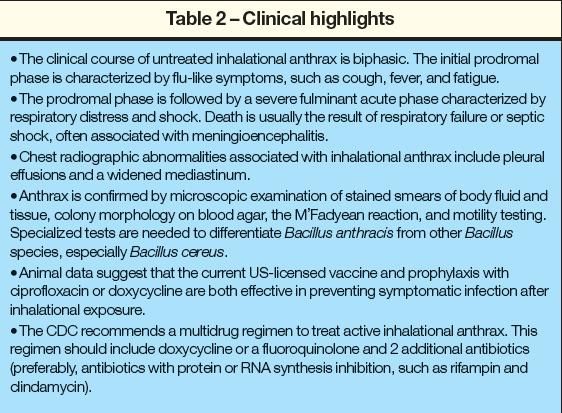
Because most clinicians are unlikely to have encountered a patient with anthrax, it is difficult to remain vigilant for the signs of such a rare illness (Table 2). Ongoing efforts to improve clinician knowledge of the diagnosis and treatment of anthrax and tabletop exercises and drills that prepare for the logistical challenges of providing large-scale prophylaxis and treatment are critical in reducing mortality.
References:
REFERENCES
1.
Brachman PS, Gold H, Plotkin SA, et al. Field evaluation of a human anthrax vaccine.
Am J Public Health.
1962;52:632-645.
2.
Wright GG, Green TW, Kanode RG Jr. Studies on immunity in anthrax. V. Immunizing activity of alumprecipitated protective antigen.
J Immunol.
1954;73:387-391.
3.
Grabenstein JD. Anthrax vaccine: a review.
Immunol Allergy Clin North Am.
2003;23:713-730.
4.
Pitt ML, Little SF, Ivins BE, et al. In vitro correlate of immunity in a rabbit model of inhalational anthrax.
Vaccine.
2001;19:4768-4773.
5.
Ivins BE, Pitt ML, Fellows PF, et al. Comparative efficacy of experimental anthrax vaccine candidates against inhalation anthrax in rhesus macaques.
Vaccine.
1998;16:1141-1148.
6.
Johnson-Winegar A. Comparison of enzymelinked immunosorbent and indirect hemagglutination assays for determining anthrax antibodies.
J Clin Microbiol.
1984;20:357-361.
7.
Buchanan TM, Feeley JC, Hayes PS, Brachman PS. Anthrax indirect microhemagglutination test.
J Immunol.
1971;107:1631-1636.
8.
Pittman PR, Norris SL, Barrera Oro JG, et al. Patterns of antibody response in humans to the anthrax vaccine adsorbed (AVA) primary (six-dose) series.
Vaccine.
2006;24:3654-3660.
9.
Pittman PR, Kim-Ahn G, Pifat DY, et al. Anthrax vaccine: immunogenicity and safety of a dosereduction, route-change comparison study in humans.
Vaccine.
2002;20:1412-1420.
10.
Jernigan DB, Raghunathan PL, Bell BP, et al. Investigation of bioterrorism-related anthrax, United States, 2001: epidemiologic findings.
Emerg Infect Dis.
2002;8:1019-1028.
11.
Turnbull PC, Broster MG, Carman JA, et al. Development of antibodies to protective antigen and lethal factor components of anthrax toxin in humans and guinea pigs and their relevance to protective immunity.
Infect Immun.
1986;52:356-363.
12.
Belton FC, Darlow HM, Henderson DW. The use of anthrax antigen to immunise man and monkey.
Lancet.
1956;271:476-479.
13.
Centers for Disease Control and Prevention. Use of anthrax vaccine in response to terrorism: supplemental recommendations of the Advisory Committee on Immunization Practices.
MMWR.
2002;51:1024-1026.
14.
Nass M. The Anthrax Vaccine Program: an analysis of the CDC's recommendations for vaccine use.
Am J Public Health.
2002;92:715-721.
15.
Sartin JS. Gulf War Syndrome: the final chapter?
Mayo Clin Proc.
2006;81:1425-1426.
16.
Advisory Committee on Immunization Practices. Use of anthrax vaccine in the United States.
MMWR.
2000;49:1-20.
17.
Tierney BC, Martin SW, Franzke LH, et al; Centers for Disease Control and Prevention's Anthrax Vaccine and Antimicrobial Availability Program. Serious adverse events among participants in the Centers for Disease Control and Prevention's Anthrax Vaccine and Antimicrobial Availability Program for persons at risk for bioterrorism-related inhalational anthrax.
Clin Infect Dis.
2003;37:905-911.
18.
Institute of Medicine. In: Joellenbeck LM, Zwanziger LL, Durch JS, Strom BL, eds.
The Anthrax Vaccine: Is It Safe? Does It Work?
Washington, DC: National Academy Press; 2002.
19.
Friedlander AM, Welkos SL, Pitt ML, et al. Postexposure prophylaxis against experimental inhalation anthrax.
J Infect Dis.
1993;167:1239-1243.
20.
Heine HS, Bassett J, Miller L, et al. Determination of antibiotic efficacy against Bacillus anthracis in a mouse aerosol challenge model.
Antimicrob Agents Chemother.
2007;51:1373-1379.
21.
Steward J, Lever MS, Simpson AJ, et al. Postexposure prophylaxis of systemic anthrax in mice and treatment with fluoroquinolones.
J Antimicrob Chemother.
2004;54:95-99.
22.
Jefferds MD, Laserson K, Fry AM, et al. Adherence to antimicrobial inhalational anthrax prophylaxis among postal workers, Washington, DC, 2001.
Emerg Infect Dis.
2002;8:1138-1144.
23.
Baggett HC, Rhodes JC, Fridkin SK, et al. No evidence of a mild form of inhalational Bacillus anthracis infection during a bioterrorism-related inhalationalanthrax outbreak in Washington, DC, in 2001.
Clin Infect Dis.
2005;41:991-997.
24.
Brookmeyer R, Blades N. Prevention of inhalational anthrax in the US outbreak.
Science.
2002;295:1861.
25.
Doolan DL, Freilich DA, Brice GT, et al. The US capitol bioterrorism anthrax exposures: clinical epidemiological and immunological characteristics.
J Infect Dis.
2007;195:174-184.
26.
Bravata DM, Zaric GS, Holty JE, et al. Reducing mortality from anthrax bioterrorism: strategies for stockpiling and dispensing medical and pharmaceutical supplies.
Biosecur Bioterror.
2006;4:244-262.
27.
Bell DM, Kozarsky PE, Stephens DS. Clinical issues in the prophylaxis, diagnosis, and treatment of anthrax.
Emerg Infect Dis.
2002;8:222-225.
28.
Brookmeyer R, Johnson E, Bollinger R. Modeling the optimum duration of antibiotic prophylaxis in an anthrax outbreak.
Proc Natl Acad Sci U S A.
2003;100:10129-10132.
29.
Glassman HN. Discussion.
Bacteriol Rev.
1966;30:657-659.
30.
Vietri NJ, Purcell BK, Lawler JV, et al. Shortcourse postexposure antibiotic prophylaxis combined with vaccination protects against experimental inhalational anthrax.
Proc Natl Acad Sci U S A.
2006;103:7813-7816.
31.
Fowler RA, Sanders GD, Bravata DM, et al. Cost-effectiveness of defending against bioterrorism: a comparison of vaccination and antibiotic prophylaxis against anthrax.
Ann Intern Med.
2005;142:601-610.
32.
Schmitt B, Dobrez D, Parada JP, et al. Responding to a small-scale bioterrorist anthrax attack: cost-effectiveness analysis comparing preattack vaccination with postattack antibiotic treatment and vaccination.
Arch Intern Med.
2007;167:655-662.
33.
Holty JE, Bravata DM, Liu H, et al. Systematic review: a century of inhalational anthrax cases from 1900 to 2005.
Ann Intern Med.
2006;144:270-280.
34.
Centers for Disease Control and Prevention. Update: investigation of bioterrorism-related anthrax and interim guidelines for exposure management and antimicrobial therapy, October 2001.
MMWR.
2001;50:909-919.
35.
Sclavo A. Sullo stato presente della sieroterapia anticarbonchiosa.
Rivista di Igiene e Sanita Pubblica.
1903;14:519-587.
36.
Inglesby TV, O'Toole T, Henderson DA, et al. Anthrax as a biological weapon, 2002: updated recommendations for management.
JAMA.
2002;287:2236-2252.
37.
Casadevall A. Passive antibody administration (immediate immunity) as a specific defense against biological weapons.
Emerg Infect Dis.
2002;8:833-841.
38.
Greenfield RA, Bronze MS. Prevention and treatment of bacterial diseases caused by bacterial bioterrorism threat agents.
Drugs Discov Today.
2003;8:881-888.
39.
Rainey GJ, Young JA. Antitoxins: novel strategies to target agents of bioterrorism.
Nat Rev Microbiol.
2004;2:721-726.
40.
Welkos S, Little S, Friedlander A, et al. The role of antibodies to Bacillus anthracis and anthrax toxin components in inhibiting the early stages of infection by anthrax spores.
Microbiology.
2001;147:1677-1685.
41.
Cote CK, Rossi CA, Kang AS, et al. The detection of protective antigen (PA) associated with spores of Bacillus anthracis and the effects of anti-PA antibodies on spore germination and macrophage interactions.
Microb Pathog.
2005;38:209-225.
42.
Knudson GB. Treatment of anthrax in man: history and current concepts.
Mil Med.
1986;151:71-77.
43.
Sherer K, Li Y, Cui X, Eichacker PQ. Lethal and edema toxins in the pathogenesis of Bacillus anthracis septic shock: implications for therapy.
Am J Respir Crit Care Med.
2007;175:211-221.
44.
Karginov VA, Robinson TM, Riemenschneider J, et al. Treatment of anthrax infection with combination of ciprofloxacin and antibodies to protective antigen of Bacillus anthracis.
FEMS Immunol Med Microbiol.
2004;40:71-74.
45.
Mair M. Brief Report: recent progress in biodefense countermeasure development.
Biosecur Bioterror.
2005;3:280-285.
46.
Walsh JJ, Pesik N, Quinn CP, et al. A case ofnaturally acquired inhalation anthrax: clinical care and analyses of anti-protective antigen immunoglobulin G and lethal factor.
Clin Infect Dis.
2007;44:968-971.
47.
Hupert N, Bearman GM, Mushlin AI, Callahan MA. Accuracy of screening for inhalational anthrax after a bioterrorist attack.
Ann Intern Med.
2003;139:337-345.
48.
Kuehnert MJ, Doyle TJ, Hill HA, et al. Clinical features that discriminate inhalational anthrax from other acute respiratory illnesses.
Clin Infect Dis.
2003;36:328-336.
49.
Kyriacou DN, Stein AC, Yarnold PR, et al. Clinical predictors of bioterrorism-related inhalational anthrax.
Lancet.
2004;364:449-452.
50.
Cinti SK, Saravolatz L, Nafziger D, et al. Differentiating inhalational anthrax from other influenzalike illnesses in the setting of a national or regional anthrax outbreak.
Arch Intern Med.
2004;164:674-676.
51.
Fine AM, Wong JB, Fraser HS, et al. Is it influenza or anthrax? A decision analytic approach to the treatment of patients with influenza-like illnesses.
Ann Emerg Med.
2004;43:318-328.
52.
Mayer TA, Morrison A, Bersoff-Matcha S, et al. Inhalational anthrax due to bioterrorism: would current Centers for Disease Control and Prevention guidelines have identified the 11 patients with inhalational anthrax from October through November 2001?
Clin Infect Dis.
2003;36:1275-1283.
53.
Centers for Disease Control and Prevention. Investigation of bioterrorism-related anthrax and interim guidelines for clinical evaluation of persons with possible anthrax.
JAMA.
2001;286:2392-2396.
54.
Kyriacou DN, Yarnold PR, Stein AC, et al. Discriminating inhalational anthrax from communityacquired pneumonia using chest radiograph findings and a clinical algorithm.
Chest.
2007;131:489-496.
55.
Howell JM, Mayer TA, Hanfling D, et al. Screening for inhalational anthrax due to bioterrorism: evaluating proposed screening protocols.
Clin Infect Dis.
2004;39:1842-1847.
56.
Kaufman Z, Aharonowitz G, Dichtiar R, Green MS. Estimating the usual prevalence and incidence of acute illness in the community: implications for pandemic influenza and bioterrorism preparedness.
Isr Med Assoc J.
2006;8:563-567.
57.
Buehler JW, Berkelman RL, Hartley DM, Peters CJ. Syndromic surveillance and bioterrorism-related epidemics.
Emerg Infect Dis.
2003;9:1197-1204.
58.
Loonsk JW. BioSense-a national initiative for early detection and quantification of public health emergencies.
MMWR.
2004;53(suppl):53-55.
59.
Buckeridge DL, Owens DK, Switzer P, et al. Evaluating detection of an inhalational anthrax outbreak.
Emerg Infect Dis.
2006;12:1942-1949.
60.
Braithwaite RS, Fridsma D, Roberts MS. The cost-effectiveness of strategies to reduce mortality from an intentional release of aerosolized anthrax spores.
Med Decis Making.
2006;26:182-193.
61.
Inglesby T, Grossman R, O'Toole T. A plague on your city: observations from TOPOFF.
Clin Infect Dis.
2001;32:436-445.
62.
Koplan J. CDC's strategic plan for bioterrorism preparedness and response.
Public Health Rep.
2001;116(suppl 2):9-16.
63.
Terriff CM, Tee AM. Citywide pharmaceutical preparation for bioterrorism.
Am J Health Syst Pharm.
2001;58:233-237.
64.
Case GG, West BM, McHugh CJ. Hospital preparedness for biological and chemical terrorism in central New Jersey.
N J Med.
2001;98:23-33.
65.
Centers for Disease Control and Prevention. Statement by the Department of Health and Human Services Regarding Additional Options for Preventive Treatment for Those Exposed to Inhalation Anthrax. Tuesday, Dec 18, 2001.
http://www.bt.cdc.gov/documentsapp/Anthrax/12182001/hhs12182001.asp
. Accessed May 6, 2008.
66.
Centers for Disease Control and Prevention. Update: interim recommendations for antimicrobial prophylaxis for children and breastfeeding mothers and treatment of children with anthrax.
MMWR.
2001;50:1014-1016.
67.
ACOG Committee on Obstetric Practice. ACOG Committee Opinion number 268, February 2002. Management of asymptomatic pregnant or lactating women exposed to anthrax. American College of Obstetricians and Gynecologists.
Obstet Gynecol.
2002;99:366-368.
68.
Benavides S, Nahata MC. Anthrax: safe treatment for children.
Ann Pharmacother.
2002;36:334-337.
