- Clinical Technology
- Adult Immunization
- Hepatology
- Pediatric Immunization
- Screening
- Psychiatry
- Allergy
- Women's Health
- Cardiology
- Pediatrics
- Dermatology
- Endocrinology
- Pain Management
- Gastroenterology
- Infectious Disease
- Obesity Medicine
- Rheumatology
- Nephrology
- Neurology
- Pulmonology
The Future of Inhaled Insulin Therapy
Diabetes is a destructive disease that kills thousands eachyear in the United States and disables thousands more, and its incidence hasbeen rising dramatically. Glycemic control is imperative to forestallcomplications; however, it can be difficult for patients to achieve glycemicgoals.
Diabetes is a destructive disease that kills thousands each
year in the United States and disables thousands more, and its incidence has
been rising dramatically. Glycemic control is imperative to forestall
complications; however, it can be difficult for patients to achieve glycemic
goals. Many persons with diabetes will require insulin therapy to obtain
glycemic goals. Clinicians and patients are sometimes apprehensive about
starting insulin therapy for various reasons. Several inhaled insulin products
are in development, including the AIR and Technosphere Insulin systems, although
one product (Exubera) was recently removed from the market because of
disappointing sales and the development of another product (AERx) was recently
discontinued. Inhaled insulin may be an alternative therapy and because of added
convenience may improve patient adherence. (Drug Benefit Trends. 2008;20:63-70)
Diabetes is a leading cause of blindness, end-stage renal disease, neuropathies, and peripheral vascular disease, which can result in amputation. Persons with diabetes have as high a risk of a cardiovascular event as persons without diabetes who have had such an event.1,2 In addition to being the sixth leading cause of death in the United States, diabetes is a national epidemic: approximately 16 million US persons have received a diagnosis of diabetes, and an additional 6 million US persons are believed to have undiagnosed diabetes.3
Tight glycemic control is imperative to reduce the risk of complications associated with type 1 and type 2 diabetes.4-7 The American Diabetes Association recommends a glycosylated hemoglobin (A1C) level below 7% minimally, and as close to 6% as possible if this level can be achieved without significant adverse effects.8 Glycemic control reduces the risk of microvascular disease (retinopathy, neuropathy, nephropathy) and shows a trend to reduce the risk of macrovascular events.9 However, glycemic control is difficult to achieve, as evidenced in clinical trials4-6 and in clinical practice.10,11 Insulin therapy is required to meet these goals in many patients, but multiple daily injections of insulin are perceived adversely by patients and clinicians alike.12,13
Insulin is a polypeptide that is denatured in the GI tract when taken orally; therefore, intravenous or subcutaneous administration is necessary. Patients are required to receive multiple doses of insulin parenterally. Since the advent of insulin therapy, alternative delivery routes and systems have been investigated. Pulmonary delivery is ideal because insulin can diffuse into the bloodstream at the level of the alveoli.
In 2006, Exubera (Pfizer), an inhaled dry powder formulation of fast-acting insulin, was approved for marketing by the FDA in the United States and by the European Agency for the Evaluation of Medicinal Products in Europe for treating adults with type 1 or type 2 diabetes.14 However, in October 2007, Pfizer removed Exubera from the market because of poor sales. In January 2008, Novo Nordisk announced that it would discontinue all further development of the AERx system; the company stated that the decision was not due to safety concerns. Other inhaled insulin systems are in different stages of development (Table 1).
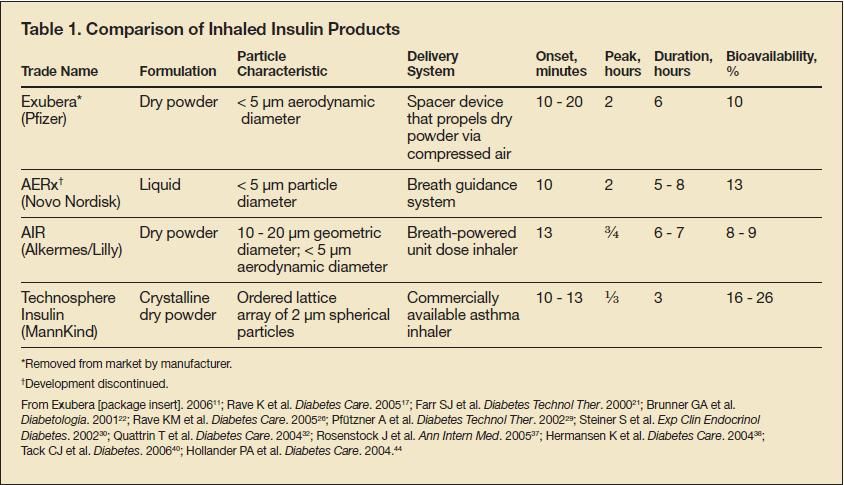
Pulmonary Delivery of Insulin
Pulmonary delivery of insulin can be considered ideal because of the large surface area of the lung available for absorption (up to 100 m2), the high permeability of the alveolar surface, and the vast circulation. However, most airborne particles are deposited in the upper airways, where the mucociliary elevator expels them from the lungs into the GI tract. In order for a substance to reach the alveoli, it must have certain characteristics, including appropriate size, particle density, and morphology.8,10 Particles of 1 to 3 μm are ideal for penetration to the alveoli and deposit minimally in the oropharynx.8 Particles smaller than 1 μm are mostly exhaled, and those larger than 10 μm tend to deposit in the oropharynx.8
Substances that do not have the appropriate particle density or morphology are removed by macrophages.8 Small, nonporous particles are engulfed by macrophages quickly, whereas porous particles are removed at a slower rate.8,10 The mode of delivery to the agent’s target can also hinder therapy administered through the pulmonary delivery system.15 Many delivery systems propel particles with too much velocity, depositing the substance in the oropharynx rather than the lower airways.8 Inhaler technology is designed to provide dosing while minimizing losses within the device and in the environment. In addition, advances in particle technology have improved reproducibility and enhanced delivery to the deep lung.
Pharmacokinetics and Glucodynamics
Each pulmonary insulin delivery system consists of 3 components: an insulin formulation, unique insulin delivery “packaging,” and a delivery device.
Exubera system. Although Pfizer recently stopped selling Exubera, it is important to discuss this system, since it was the first FDA-approved nonparenteral inhaled insulin product.11 Exubera was available in a short-acting formulation to control postprandial glucose excursions. The delivery system administered a dry powder through a device that provided a spacer into which particles were launched via a compressed air supply and then slowly inhaled by the patient.11 The aerosolized insulin powder was contained in blister packs that were administered through the inhaler.
Each blister pack contained either 1 or 3 mg of insulin. The 1-mg blister pack was equivalent to approximately 3 units of SC insulin, and the 3-mg blister pack was equivalent to approximately 8 units of SC insulin. The Exubera system was designed to emit a specific dose of insulin from the mouthpiece (Table 2).11 Up to 45% of the 1-mg blister pack contents and 25% of the 3-mg blister pack contents were retained within the blister pack well.11 As a result, patients needed to dose Exubera carefully because the contents of three 1-mg blister packs did not equal that of one 3-mg blister pack. When combined, three 1-mg blister packs had a 30% to 40% higher maximum serum concentration (Cmax) of insulin after a dose of insulin and area under the curve (AUC) compared with the Cmax value and AUC of one 3-mg blister pack.11
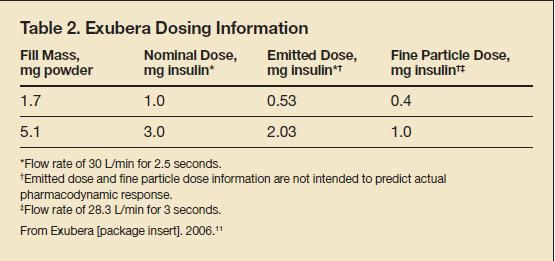
Serum insulin concentrations in persons with type 1 or type 2 diabetes peaked faster with Exubera (49 minutes; range, 30 to 90 minutes) than with SC regular insulin (105 minutes; range, 60 to 240 minutes).11 In persons with type 2 diabetes who received a single dose of Exubera (6 mg) and a single dose of SC regular insulin (18 units) on separate days, insulin reached peak serum concentration more rapidly after inhalation of Exubera (43 minutes) than after injection of SC regular insulin (120 minutes).11 In healthy volunteers, onset of glucose-lowering activity occurred in 31 minutes; the maximum glucose-lowering effect occurred at approximately 110 minutes.16 The duration of action was 6 hours, which was longer than that of SC insulin lispro and comparable to that of SC regular insulin.16 When compared with SC regular insulin, Exubera had a faster onset of action (32 vs 48 minutes, respectively; P = .001).17
A similar result was reported when Exubera was compared with SC insulin lispro (32 vs 41 minutes, respectively; P < .05).17 The time to maximal metabolic effect of Exubera was similar to that of SC insulin lispro (143 vs 137 minutes, respectively; P = not significant [NS]), but shorter when compared with that of SC regular insulin (193 minutes; P < .01).
The duration of metabolic activity of Exubera was similar to that of SC regular insulin but significantly longer than the duration of SC insulin lispro. The reproducibility of Exubera was similar to that of SC regular insulin, and the intrasubject differences between the 2 same-dose routes of administration were small.18,19
Special populations and influence of absorption. As with most pharmacotherapeutic agents, there are special populations in whom the absorption of insulin is affected. This is particularly true for persons with chronic lung conditions, such as chronic obstructive pulmonary disease (COPD) and asthma, and for persons who are current or former smokers.
Underlying lung disease. Exubera was not studied in certain patient populations, such as persons with diabetes who had comorbid asthma or COPD. Plasma insulin levels in such patients with comorbid COPD were 2 times as high as those in such patients without comorbid COPD.11 Studies have consistently reported an approximate reduction of 20% in the absorption of inhaled insulin in participants with mild to moderate asthma compared with that of participants without asthma. These significant absorption differences and the lack of data provide the basis for the current recommendation against the use of inhaled insulin by persons with diabetes and comorbid asthma or COPD.11
Smoking. Because smoking more than doubles insulin levels,20 it was recommended that persons with diabetes who smoke or stopped smoking in the preceding 6 months not use Exubera.11 Within 1 week of smoking cessation, the bioavailability of Exubera in study participants decreased by approximately 50% compared with that of smokers; however, this was still higher than that of nonsmokers. These rapid changes in absorption make dose prediction, as well as the potential risk of hypoglycemia, higher in smokers.11,20
Comorbid respiratory tract illness. Clinically, comorbid uncomplicated upper respiratory tract illnesses (bronchitis, upper respiratory tract infection, rhinitis) did not affect rates of glycemic control or hypoglycemia in persons with diabetes.11 About 1 in 20 study participants temporarily discontinued Exubera therapy during the illness; an alternative therapy was to be administered.11
AERx system. The development of the AERx insulin diabetes management system was recently discontinued by Novo Nordisk; however, the system is important to review because it used a microprocessor-controlled inhaler device that monitored the breathing pattern of patients and actuated when a combination of breathing parameters fell to a predetermined range.15,21 A fine mist was created by driving the insulin solution through a single-use nozzle.21 The handheld inhaler was about the size of a small paperback book and had a slot into which a disposable insulin strip was inserted. A single-use nozzle on the insulin strip contained hundreds of 1-μm holes that uniformly released the aerosol insulin.20,21
For dosing, the AERx system used units, each of which provided the same glucose-lowering effect as 1 IU of SC insulin.21,22 The insulin was supplied in a strip that contained 10 IU. A patient was able to receive between 2 and 10 IU of insulin with each strip, in 1-IU increments.21,22
Onset of action with the AERx system was approximately 10 minutes, with peak insulin concentrations occurring about 1 hour after inhalation.22 Dose affected the duration of action: a dose of 0.3 IU/kg lasted 5 hours, and a dose of 1.8 IU/kg lasted 8 hours.22 The AERx system demonstrated a rapid absorption of insulin and a corresponding faster decline in plasma glucose compared with those of SC insulin administration.21 Intrapatient variability was similar in persons with type 1 diabetes who were given equivalent doses of SC regular insulin or inhaled insulin.23 The speed of absorption and resulting hypoglycemic activity were determined by the patients’ volume and depth of breath, hence the microprocessor-controlled inhaler design.21
Results of studies of special populations (persons with diabetes who smoke or have underlying lung disease, comorbid asthma, or comorbid respiratory tract infection) who used the AERx system mirrored those of persons who used Exubera. Smokers’ insulin AUC was 60% higher, their Cmax value was 3 times higher, and their absorption was more rapid than those of nonsmokers.20 Studies of persons with asthma showed decreased inhaled insulin absorption compared with that of healthy study participants without asthma.24 In addition to the reduced absorption, persons with asthma had smaller reductions in blood glucose levels, so they might require either higher doses of insulin to achieve glycemic control or bronchodilator therapy before each dose.24 In persons with comorbid respiratory tract infections, insulin pharmacokinetics were not found to be significantly different during or immediately after acute infection.25
AIR system. The AIR system (Alkermes/Lilly) uses a semidisposable inhaler to deliver a dry powder insulin particle characterized by large size but low density, thereby keeping the aerodynamic diameter small. Currently under development are 2-unit and 6-unit capsules. The inhaler is approximately the size of a cell phone and uses breath-actuated technology to deliver the dry powder.8,26 An insulin capsule is placed in the inhaler, where it is punctured.8 The inhalation of air through the device provides enough energy to spin the capsule, forming an aerosol for inhalation.
When compared with SC insulin lispro in healthy participants, the AIR system appeared to be similar in terms of pharmacokinetic and pharmacodynamic parameters.26 The AIR system demonstrated an onset of action of about 13 minutes, which was significantly faster than that of SC insulin lispro. With the AIR system, peak serum concentrations were reached in 45 minutes, 30 minutes, and 45 minutes for 2.6-mg, 5.2-mg, and 7.8-mg doses, respectively. For study participants who received a 7.8-mg dose, the duration of action was 8 hours compared with 6 to 7 hours for participants who received the 2.6-mg and 5.2-mg doses. Intrasubject variability of the AIR system is similar to that of SC insulin lispro.26
A significant difference between the Exubera system and the AIR system is the capsule dose equivalency. Unlike the lack of equivalence between Exubera’s blister packs, 3 inhalations of a 2-unit AIR capsule is equivalent to 1 inhalation of a 6-unit capsule.
The pharmacokinetics and glucodynamics of the AIR system have been evaluated in persons with comorbid COPD.27,28 Their mean AUC and metabolic effect were reduced compared with those of healthy persons. In addition, the intrasubject variability was greater in persons with chronic bronchitis.27 Participants with comorbid COPD were found to have a small decrease in forced expiratory volume in 1 second (FEV1), but this is not clinically significant.28
Technosphere Insulin system. The Technosphere Insulin system (MannKind) is unique in that it captures and stabilizes peptides in small particles.29 A small organic molecule, 3,6-bis[N-fumaryl-N-(nbutyl) amino]-2,5-diketopiperazine, self-assembles in a mild acidic environment into microspheres of 2 to 3 μm.30 Peptides in the reaction solution, such as insulin, are trapped in the microspheres during self-assembly. After drying, the particles are suitable for pulmonary delivery. In the neutral pH of the deep lungs, the microspheres dissolve and facilitate the absorption of insulin into the systemic circulation. The carrier molecules are excreted as ammonium salts in the urine within hours.29 The Technosphere Insulin system is provided in gelatin capsules that contain 50 IU of dry powder regular human insulin.30
In studies evaluating the technology, an inhaler specifically adapted to the physical properties of Technosphere Insulin was used.29 The inhaler does not require any external energy, and study participants were instructed to take 3 deep breaths per capsule. Delivery of the drug is determined by the patients’ volume and depth of breath.30
In a euglycemic glucose clamp technique study evaluating the biologic efficacy and pharmacokinetic properties of the Technosphere Insulin technology,30 5 healthy, nonsmoking participants were randomized to 1 of 3 groups: those who inhaled 100 IU of Technosphere Insulin, those who received 10 IU of SC regular insulin, and those who received 5 IU of IV regular insulin. Participants in the Technosphere Insulin group showed an onset of action (13 minutes) similar to that of those in the IV regular insulin group (9 minutes) and much faster than that of those in the SC regular insulin group (121 minutes).30 Insulin concentrations of the participants in the Technosphere Insulin group returned to baseline after 3 hours.
Efficacy of Inhaled Insulin Systems
Exubera system. The efficacy of Exubera was evaluated in more than 4000 persons with type 1 or type 2 diabetes. (It should be noted that most of the clinical trials began before the introduction of the basal insulin glargine or detemir.)
Type 1 diabetes. In a proof-of-concept trial of 72 persons with type 1 diabetes,31 a similar reduction in A1C levels was achieved when participants were randomized to twice-daily isophane insulin in combination with preprandial Exubera versus once-daily ultralente insulin in combination with preprandial SC regular insulin. In addition, 328 persons with type 1 diabetes and a baseline A1C level of approximately 8.2% received preprandial Exubera or SC regular insulin in combination with neutral protamine Hagedorn (NPH) insulin before breakfast and at bedtime. After 24 weeks, those in the Exubera group had an average A1C level of 7.7% (±0.1%), while those in the SC regular insulin group had an average A1C level of 7.8% (±1.2%). The 2 treatment regimens had similar results for A1C level reductions and the number of participants whose A1C levels fell below 7.0%.31
Quattrin and colleagues32 randomly assigned 335 persons with type 1 diabetes with a baseline A1C level of approximately 8.3% to either preprandial Exubera and ultralente at bedtime or NPH insulin twice daily in combination with 2 regular insulin doses before breakfast and the evening meal for 24 weeks. Exubera and SC insulin reduced the average A1C level to 7.9% (±1.1%) and 7.7% (±0.9%) (adjusted treatment group difference, 0.16%; 95% confidence interval [CI],–0.01 to 0.32), respectively. In addition, a comparable number of patients achieved A1C levels below 7% (15.9% and 15.5%, respectively). The fasting plasma glucose (FPG) level of patients receiving Exubera (baseline FPG level, 194 mg/dL) was reduced more than the FPG level in patients receiving SC insulin (baseline FPG level, 203 mg/ dL) (–25.2 mg/dL; 95% CI, –43.4 to –7.0 mg/dL).
Type 2 diabetes. Weiss and coworkers33 evaluated the effect of the addition of Exubera or the continuation of oral antihyperglycemic agents (OHAs) on glycemic control in 68 persons with type 2 diabetes with an average baseline A1C level of approximately 9.8% who were taking near maximal or maximal therapeutic doses of a sulfonylurea and/or metformin. At 12 weeks, patients who had been randomized to receive Exubera 3 times daily before meals had an A1C level reduction of 2.3%, whereas those who had continued taking their OHAs had only a 0.1% A1C level reduction (P < .001). Of the participants taking Exubera, 11 (34%) achieved an A1C level of less than 7%; no patients continuing to take OHAs achieved this level.
DeFronzo and colleagues34 randomized 145 persons with type 2 diabetes with poor glycemic control, with an average baseline A1C level of approximately 9.5%, to receive preprandial Exubera (average dosage at the end of the study, 15.3 mg/d) or rosiglitazone (average dosage, 4 mg bid). After 12 weeks, the average A1C level was reduced more significantly in the Exubera group than in the rosiglitazone group (–2.3% vs –1.4%, respectively; P < .0001). In addition, significantly more patients taking Exubera achieved an A1C level of less than 7% (44%) or 6.5% or less (28%) than those taking rosiglitazone (18% and 7.5%, respectively).
Barnett and associates35,36 conducted 2 open-label, parallel, 24-week trials of persons with type 2 diabetes. In the first study, 423 patients whose diabetes was poorly controlled with sulfonylurea monotherapy-their average baseline A1C level was 9.7%-were randomized to the addition of preprandial Exubera or metformin.35 In patients taking near-maximal therapeutic doses of failing sulfonylureas, the addition of Exubera (average dosage, 12.1 mg/d) lowered A1C levels more than the addition of metformin (average dosage, 1 g bid) (2.06% vs 1.83%, respectively; P = .014). Of the participants taking Exubera, 54 (25%) achieved an A1C level of 7.0% or less, whereas 45 participants (23%) taking metformin reached this level (P = NS).
In the second study, 476 participants whose diabetes was inadequately controlled with metformin monotherapy (average dosage, more than 1.5 g/d for 2 or more months) were randomized to the addition of preprandial Exubera (average dosage, 13.2 mg/d) or a sulfonylurea (glibenclamide [glyburide in the United States], average dosage, 7.6 mg/d).36 The A1C levels of the patients, whose average baseline A1C level was 9.5%, were reduced with Exubera and glibenclamide (2.03% and 1.88%, respectively; P = NS). Participants were much more likely to achieve an A1C level of 7.0% or less with Exubera if their baseline A1C level was below 9.5%. Overall, Exubera proved to be noninferior to metformin or glibenclamide in participants whose baseline A1C level was below 9.5%, and superior to the addition of metformin or sulfonylurea in those whose baseline A1C level was greater than 9.5%.
Rosenstock and colleagues37 conducted an open-label, multicenter study of 309 persons with type 2 diabetes whose average baseline A1C level was approximately 9.3%. The patients were randomized to (1) continued current OHAs; (2) preprandial Exubera as monotherapy (discontinuation of all OHAs); or (3) current OHA therapy and Exubera. After 12 weeks, A1C level reductions in the 3 groups were 0.2%, 1.4%, and 1.7%, respectively. Achieving A1C levels of less than 7.0% was significantly greater in participants who received Exubera monotherapy (17%) or the addition of Exubera to OHAs (32%) compared with patients who continued to receive OHAs (1%). The mean dosage of Exubera was higher with monotherapy (26.4 mg/d) than with the continuation of OHAs (13.1 mg/d).
Exubera versus SC insulin regimens. In a 12-week trial,38 26 persons with type 2 diabetes who were treated with insulin and not taking OHAs were randomized either to continue their conventional insulin regimen (19 IU of regular insulin and 51 IU of long-acting insulin) or to start preprandial Exubera (average dosage, 14.6 mg/d) with a single bedtime injection of ultralente insulin (average dosage, 35.7 IU/d). The A1C levels of participants in the Exubera group, whose average baseline A1C level was 8.67%, declined by 0.71%; the A1C levels of those in the conventional insulin regimen group remained unchanged.
AERx system. Clinical data are limited, but in a 12-week study of persons with type 2 diabetes (average baseline A1C level, 8.5%),38 a regimen of 3 AERx insulin inhalations in conjunction with bedtime NPH produced a reduction in A1C levels (0.69 ± 0.77%) comparable to that of fast-acting SC human insulin given before each meal (0.77 ± 0.77%). No statistically significant difference between the 2 groups was seen for FPG or postprandial glucose readings.
AIR system. As with the AERx system, clinical data on the AIR system is limited. In a 12-week study, persons with type 1 diabetes were randomized to receive prandial SC insulin or AIR inhaled insulin in conjunction with glargine insulin. The reduction in A1C levels with AIR inhaled insulin was comparable to that of SC injected insulin.39
Technosphere Insulin system. Clinical data on the Technosphere Insulin system are also limited. A study of 227 persons whose type 2 diabetes was poorly controlled showed that the A1C levels of those given prandial Technosphere Insulin at dosages of 28, 42, and 56 IU for 12 weeks in conjunction with insulin glargine declined by 0.67%, 0.70%, and 0.78%, respectively.40
Safety of Inhaled Insulin Systems
Pulmonary function parameters. FEV1 and carbon monoxide–diffusing capacity (DLCO) are parameters used to evaluate lung function in studies testing inhaled insulin products. FEV1 values are reported as a percentage of predicted normal values and are used to measure airway obstructions or changes in airway diameter. DLCO indicates oxygen-diffusing capacity influenced by lung surface area, speed of blood flow, and thickness of the air-blood barrier. A reduction in DLCO is a hallmark finding in persons with lung disease. Persons with diabetes have an unexplained decrease in lung function compared with those without diabetes,41,42 which is similar to the difference seen between smokers and persons who never smoked.
Pulmonary function test results. In persons with type 1 or type 2 diabetes who used Exubera, cough was reported more frequently (29.5% and 21.9%, respectively) compared with controls (10%).11 The cough was generally mild and rarely productive and accounted for 1.2% discontinuation in clinical trials.11 In addition, the cough occurred within seconds to minutes of dosing and was not associated with declines in lung function or bronchoconstriction.43
All studies on the effect of inhaled insulin on pulmonary function showed a small decline from baseline levels and found the effect to be comparable to the effect of SC insulin.31,32,34,38,39,44,45 In studies on the effect of Exubera, persons with type 1 diabetes who received inhaled insulin experienced a statistically significant decline in DLCO and a nonsignificant decrease in FEV1 compared with those randomized to receive SC insulin.31,32,43 Although there was a statistically significant decrease in DLCO within 2 weeks of initiation of inhaled insulin, it was nonprogressive.43 There were no clinical manifestations, and the decrease in DLCO was clinically insignificant.31,32 In addition, persons with type 1 or type 2 diabetes who did not receive inhaled insulin had a small decline in lung function over time.43 Studies on the use of Exubera and the AERx and AIR systems suggest that changes in lung function are not clinically significant and are reversible on treatment discontinuation.31,38,39,44
In the first long-term trial on the safe use of Exubera, Skyler and associates46 monitored 580 persons with type 1 diabetes for 2 years. The investigators found a statistically significant decrease in FEV1 within the first 3 months of therapy in subjects randomized to receive Exubera compared with those who received SC insulin; however, this decline did not persist throughout the remainder of the study. Thoracic high-resolution CT scans from participants who took Exubera for 2 years did not show any visual damage to lung tissue. In addition, the rate of pulmonary fibrosis did not increase with the use of Exubera.
Safety monitoring. Prescribing information for Exubera recommended that all persons with type 1 or type 2 diabetes have baseline spirometry (FEV1) testing, a follow-up pulmonary function assessment 6 months after initiation of treatment, and annual assessments thereafter.11 Exubera was not recommended for persons with baseline FEV1 or DLCO levels lower than 70% predicted.11 In persons who experienced a 20% or more decrease in FEV1 levels, pulmonary function testing should be repeated.11
Insulin antibody levels. Delivery of insulin to the pulmonary mucosa could induce an immune or allergic reaction, which may have safety implications. A large meta-analysis of Exubera showed higher insulin antibody levels with inhaled insulin than with comparator SC therapies.47 One clinical study found a similar result with the AERx system.38 These increases in insulin antibody levels had no statistically significant effect on insulin pharmacokinetics, glucodynamics, glucose control, or safety profile.47 No insulin antibodies were observed with the Technosphere Insulin system.37 No data are available for the AIR system.
Hypoglycemia. As with SC insulin therapy, inhaled insulin may produce hypoglycemic reactions. The nature, frequency, and severity (minor and major) of hypoglycemia in persons with type 1 or type 2 diabetes receiving inhaled insulin are similar to those in patients receiving SC insulin.31,32,37-39,44 In persons with type 2 diabetes, Exubera was associated with an increased risk of hypoglycemia when compared with metformin or rosiglitazone therapy.34,35 Hypoglycemic rates associated with the use of inhaled insulin were similar to those resulting from the addition of glibenclamide, but slightly less when compared with those of an SC regular insulin or NPH insulin regimen.36 Persons using the AIR system had increased rates of nocturnal hypoglycemia.41 Hypoglycemic rates have not been reported in persons with type 1 or type 2 diabetes receiving Technosphere Insulin.
Weight gain. In persons with type 1 diabetes, the use of Exubera resulted in a small weight gain (1 to 1.5 kg), which is similar to that associated with SC insulin regimens. Most studies of persons with type 2 diabetes showed that the use of Exubera resulted in a weight gain of approximately 2 to 3 kg. This increase was similar to that seen with the initiation of glibenclamide36 but higher than the weight loss (0.1 kg) resulting from the addition of metformin.35 Patients taking Exubera and ultralente had significantly less weight gain (0.6 kg) than patients taking regular insulin/NPH (1.4 kg).44
Conclusion
Inhalation of insulin is a new method of insulin delivery. Many persons with type 1 or type 2 diabetes fear injections and would prefer alternative methods of insulin delivery. Inhaled insulin therapy offers additional options for clinicians to improve glycemic control in patients. Exubera was the most extensively studied; however, Pfizer removed it from the market in October 2007 because of poor acceptance by patients and prescribers. Specific reasons why Exubera was not accepted are unclear. One possibility is Exubera’s dosing, which was in milligrams rather than in the units through which SC insulin is dosed. The Exubera inhalation device was 6 in long when closed and 12 in long when extended, which made portability difficult for some patients. Persons with underlying lung disease were prohibited from using inhaled insulin, which may limit the number of patients who would benefit from inhaled insulin therapy.
Before Exubera’s removal from the market, several publications described its pharmacokinetics, pharmacodynamics, and clinical efficacy. Onset and peak effect associated with the use of Exubera were similar to those of rapid-acting insulin, such as lispro, aspart, and glulisine.17,19,34,46 Exubera was also shown to effectively reduce A1C levels in persons with type 1 or type 2 diabetes.48 Ceglia and colleagues48 have suggested that inhaled insulin therapy provides the same glycemic control as SC insulin but better control than OHAs. The risk of hypoglycemia associated with Exubera was similar to that of SC regular insulin.
Safety data on the use of Exubera were compiled for 2 years, and although small changes in FEV1 and DLCO levels were observed, they were found to be reversible on treatment discontinuation.31 Despite Exubera’s removal from the market and the discontinuation of the development of the AERx system, other insulin inhalation systems, such as AIR and Technosphere Insulin, are in development, and alternative delivery of peptides continues to be an active area of research.
References:
References
- 1. Haffner SM, Lehto S, Rönnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229-234.
- 2. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143-3421.
- 3. Centers for Disease Control and Prevention. National Diabetes Fact Sheet. United States, 2005. General Information. Available at: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2005.pdf. Accessed January 8, 2008.
- 4. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977-986.
- 5. Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28:103-117.
- 6. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group [published correction appears in Lancet. 1999;354:602]. Lancet. 1998;352:837-853.
- 7. Hanefeld M, Fischer S, Julius U, et al. Risk factors for myocardial infarction and death in newly detected NIDDM: the Diabetes Intervention Study, 11-year follow-up. Diabetologia. 1996;39:1577-1583.
- 8. Valente AX, Langer R, Stone HA, Edwards DA. Recent advances in the development of an inhaled insulin product. BioDrugs. 2003;17:9-17.
- 9. American Diabetes Association. Standards of medical care in diabetes-2006. Diabetes Care. 2006;29:S4-S42.
- 10. Crowder TM, Rosati JA, Schroeter JD, et al. Fundamental effects of particle morphology on lung delivery: predictions of Stokes’ law and the particular relevance to dry powder inhaler formulation and development. Pharm Res. 2002;19:239-245.
- 11. Exubera (insulin human [rDNA origin]) inhalation powder [package insert]. New York: Pfizer Inc; 2006.
- 12. Harris MI. Health care and health status and outcomes for patients with type 2 diabetes. Diabetes Care. 2000;23:754-758.
- 13. Saaddine JB, Engelgau MM, Beckles GL, et al. A diabetes report card for the United States: quality of care in the 1990s. Ann Intern Med. 2002;136:565-574.
- 14. Lenzer J. Inhaled insulin is approved in Europe and United States. BMJ. 2006;332:321.
- 15. Farr SJ, Rowe AM, Rubsamen R, Taylor G. Aerosol deposition in the human lung following administration from a microprocessor controlled pressurised metered dose inhaler. Thorax. 1995;50:639-644.
- 16. Heinemann L, Traut T, Heise T. Time-action profile of inhaled insulin. Diabet Med. 1997;14:63-72.
- 17. Rave K, Bott S, Heinemann L, et al. Time-action profile of inhaled insulin in comparison with subcutaneously injected insulin lispro and regular human insulin. Diabetes Care. 2005;28:1077-1082.
- 18. Gelfand R, Schwartz S, Horton M. Pharmacological reproducibility of inhaled human insulin pre-meal dosing in patients with type 2 diabetes mellitus (NIDDM). Diabetes. 1998;47:A99.
- 19. Mudaliar S, Henry R, Fryburg D, et al. Within-subject variability of inhaled insulin (Exubera) versus subcutaneous regular insulin in elderly obese patients with type 2 diabetes mellitus. Diabetologia. 2003;52:A277. Abstract 802.
- 20. Himmelmann A, Jendle J, Mellen A, et al. The impact of smoking on inhaled insulin. Diabetes Care. 2003;26:677-682.
- 21. Farr SJ, McElduff A, Mather LE, et al. Pulmonary insulin administration using the AERx system: physiological and physicochemical factors influencing insulin effectiveness in healthy fasting subjects. Diabetes Technol Ther. 2000;2:185-197.
- 22. Brunner GA, Balent B, Ellmerer M, et al. Dose-response relation of liquid aerosol inhaled insulin in type I diabetic patients. Diabetologia. 2001;44:305-308.
- 23. Kapitza C, Hompesch M, Scharling B, Heise T. Intrasubject variability of inhaled insulin in type 1 diabetes: a comparison with subcutaneous insulin. Diabetes Technol Ther. 2004;6:466-472.
- 24. Henry RR, Mudaliar SR, Howland WC 3rd, et al. Inhaled insulin using the AERx Insulin Diabetes Management System in healthy and asthmatic subjects. Diabetes Care. 2003;26:764-769.
- 25. McElduff A, Mather LE, Kam PC, Clauson P. Influence of acute upper respiratory tract infection on the absorption of inhaled insulin using the AERx insulin Diabetes Management System. Br J Clin Pharmacol. 2005;59:546-551.
- 26. Rave KM, Nosek L, de la Peña A, et al. Dose response of inhaled dry-powder insulin and dose equivalence to subcutaneous insulin lispro. Diabetes Care. 2005;28:2400-2405.
- 27. Rave K, Hausmann M, de la Peña A. Pharmacokinetics (PK) and glucodynamics (GD) of human insulin inhalation powder (HIIP) in subjects with chronic obstructive pulmonary disease (COPD). Diabetes. 2006;55:A26.
- 28. Rave K, de la Peña A, Tibaldi FS, et al. AIR inhaled insulin in subjects with chronic obstructive pulmonary disease: pharmacokinetics, glucodynamics, safety, and tolerability. Diabetes Care. 2007;30:1777-1782.
- 29. Pfützner A, Mann AE, Steiner SS. Technosphere/Insulin-a new approach for effective delivery of human insulin via the pulmonary route. Diabetes Technol Ther. 2002;4:589-594.
- 30. Steiner S, Pfützner A, Wilson BR, et al. Technosphere/Insulin-proof of concept study with a new insulin formulation for pulmonary delivery. Exp Clin Endocrinol Diabetes. 2002;110:17-21.
- 31. Skyler JS, Weinstock RS, Raskin P, et al. Use of inhaled insulin in a basal/bolus insulin regimen in type 1 diabetic subjects: a 6-month, randomized, comparative trial. Diabetes Care. 2005;28:1630-1635.
- 32. Quattrin T, Bélanger A, Bohannon NJ, Schwartz SL. Efficacy and safety of inhaled insulin (Exubera) compared with subcutaneous insulin therapy in patients with type 1 diabetes: results of a 6-month, randomized, comparative trial. Diabetes Care. 2004;27:2622-2627.
- 33.Weiss SR, Cheng SL, Kourides IA, et al. Inhaled insulin provides improved glycemic control in patients with type 2 diabetes mellitus inadequately controlled with oral agents: a randomized controlled trial. Arch Intern Med. 2003;163:2277-2282.
- 34. DeFronzo RA, Bergenstal RM, Cefalu WT, et al. Efficacy of inhaled insulin in patients with type 2 diabetes not controlled with diet and exercise: a 12-week, randomized, comparative trial. Diabetes Care. 2005;28:1922-1928.
- 35. Barnett AH, Dreyer M, Lange P, Serdarevic- Pehar M. An open, randomized, parallel-group study to compare the efficacy and safety profile of inhaled human insulin (Exubera) with metformin as adjunctive therapy in patients with type 2 diabetes poorly controlled on a sulfonylurea. Diabetes Care. 2006;29:1282-1287.
- 36. Barnett AH, Dreyer M, Lange P, Serdarevic-Pehar M. An open, randomized, parallel-group study to compare the efficacy and safety profile of inhaled human insulin (Exubera) with glibenclamide as adjunctive therapy in patients with type 2 diabetes poorly controlled on metformin. Diabetes Care. 2006;29:1818-1825.
- 37. Rosenstock J, Zinman B, Murphy LJ, et al. Inhaled insulin improves glycemic control when substituted for or added to oral combination therapy in type 2 diabetes: a randomized, controlled trial. Ann Intern Med. 2005;143:549-558.
- 38. Hermansen K, Rönnemaa T, Petersen AH, et al. Intensive therapy with inhaled insulin via the AERx insulin diabetes management system: a 12-week proof-of-concept trial in patients with type 2 diabetes. Diabetes Care. 2004;27:162-167.
- 39. Garg S, Rosenstock J, Silverman BL, et al. Efficacy and safety of preprandial human insulin inhalation powder versus injectable insulin in patients with type 1 diabetes. Diabetologia. 2006;49:891-899.
- 40. Tack CJ, Boss AH, Baughman RA, et al. A randomized, double-blind placebo controlled study of the forced titration of prandial Technosphere/Insulin in patients with type 2 diabetes mellitus. Diabetes. 2006;55:A102.
- 41. Lange P, Parner J, Schnohr P, Jensen G. Copenhagen City Heart Study: longitudinal analysis of ventilatory capacity in diabetic and nondiabetic adults. Eur Respir J. 2002;20:1406-1412.
- 42. Ford ES, Mannino DM. Prospective association between lung function and the incidence of diabetes: findings from the National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Diabetes Care. 2004;27:2966-2970.
- 43. Brain JD. Unlocking the opportunity of tight glycaemic control. Inhaled insulin: safety. Diabetes Obes Metab. 2005;7(suppl 1):S14-S18.
- 44. Hollander PA, Blonde L, Rowe R, et al. Efficacy and safety of inhaled insulin (Exubera) compared with subcutaneous insulin therapy in patients with type 2 diabetes: results of a 6-month, randomized, comparative trial. Diabetes Care. 2004;27:2356-2362.
- 45. Rave K, Nosek L, Heinemann L, et al. Inhaled micronized crystalline human insulin using a dry powder inhaler: dose-response and time-action profiles. Diabet Med. 2004;21:763-768.
- 46. Skyler JS, Jovanovic L, Klioze S, et al. Two-year safety and efficacy of inhaled human insulin (Exubera) in adult patients with type 1 diabetes. Diabetes Care. 2007;30:579-585.
- 47. Fineberg SE, Kawabata T, Finco-Kent D, et al. Antibody response to inhaled insulin in patients with type 1 or type 2 diabetes. An analysis of initial phase II and III inhaled insulin (Exubera) trials and a two-year extension trial. J Clin Endocrinol Metab. 2005;90:3287-3294.
- 48. Ceglia L, Lau J, Pittas AG. Meta-analysis: efficacy and safety of inhaled insulin therapy in adults with diabetes mellitus. Ann Intern Med. 2006;145:665-675.
