- Clinical Technology
- Adult Immunization
- Hepatology
- Pediatric Immunization
- Screening
- Psychiatry
- Allergy
- Women's Health
- Cardiology
- Pediatrics
- Dermatology
- Endocrinology
- Pain Management
- Gastroenterology
- Infectious Disease
- Obesity Medicine
- Rheumatology
- Nephrology
- Neurology
- Pulmonology
Pulmonary hypertension in the elderly, part 2: Treatment
ABSTRACT: The treatment of pulmonary arterial hypertension(PAH) is directed at the underlying cause, such as diastolicheart failure or chronic thromboembolic disease. Patients withidiopathic PAH or PAH associated with connective-tissue diseasewho have World Health Organization (WHO) functionalclass II or III PAH should receive a trial of oral bosentan, ambrisentan,and/or sildenafil; inhaled iloprost is an alternative oran additive agent. If patients fail to respond to these interventionsor if they have WHO functional class IV PAH, considersubcutaneous or intravenous treprostinil or epoprostenol. Theuse of these latter agents is much more complicated and maybe difficult to initiate in elderly patients. (J Respir Dis. 2008;29(12):468-474)
ABSTRACT: The treatment of pulmonary arterial hypertension (PAH) is directed at the underlying cause, such as diastolic heart failure or chronic thromboembolic disease. Patients with idiopathic PAH or PAH associated with connective-tissue disease who have World Health Organization (WHO) functional class II or III PAH should receive a trial of oral bosentan, ambrisentan, and/or sildenafil; inhaled iloprost is an alternative or an additive agent. If patients fail to respond to these interventions or if they have WHO functional class IV PAH, consider subcutaneous or intravenous treprostinil or epoprostenol. The use of these latter agents is much more complicated and may be difficult to initiate in elderly patients. (J Respir Dis. 2008;29(12):468-474)
Pulmonary arterial hypertension (PAH) is an increasingly recognized cause of shortness of breath in elderly persons. In the November 2008 issue of The Journal of Respiratory Diseases, we reviewed the clinical presentation and diagnostic evaluation of PAH. In this article, we will focus on treatment.
TREATMENT
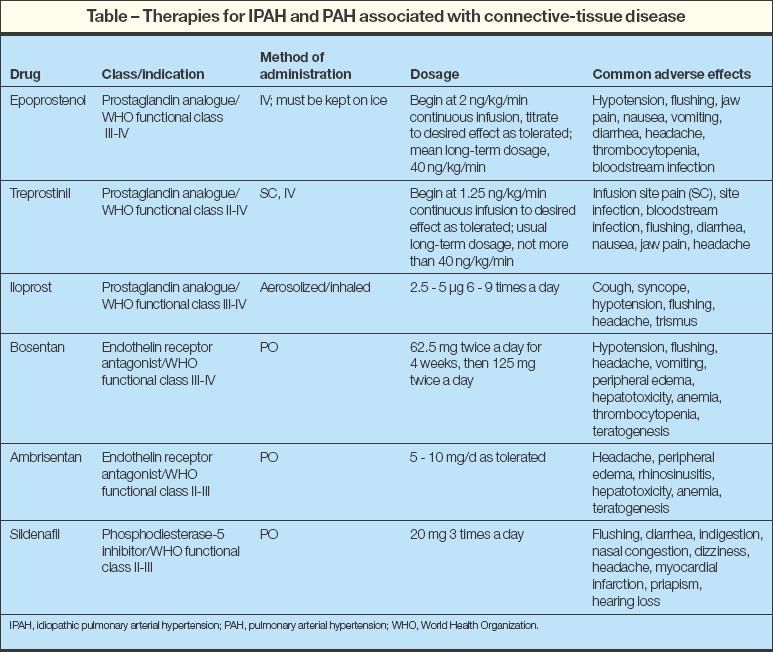
The traditional therapies for idiopathic PAH (IPAH) have included calcium channel blockers (CCBs), anticoagulants, diuretics, digoxin, and supplemental oxygen, but in the past several years, multiple treatments have emerged (Table). These therapies range from continuous intravenous medications, such as epoprostenol (an intravenous prostacyclin) and treprostinil (a subcutaneous or intravenous prostaglandin analogue), to oral or inhaled medications, including endothelin receptor antagonists (bosentan, ambrisentan), sildenafil (a phosphodiesterase type 5 inhibitor), and iloprost (an inhaled prostaglandin vasodilator). However, the approach to treatment is different in patients with diastolic heart failure and pulmonary hypertension (PH) (see below "Diastolic heart failure and pulmonary hypertension").
Very few studies in this area have differentiated elderly from young patients, so it is unclear whether treatment efficacy varies with age. The complicated nature of both diagnosis and management requires expertise in the area of PH. Therefore, when this diagnosis is suspected or confirmed, referral to a specialty center is recommended.
Traditional therapies
A vasodilator challenge is central to determining whether treatment with oral CCBs may be effective. Patients who show a positive response to vasodilator testing should be given an oral CCB trial (such as diltiazem, nifedipine, or amlodipine). Patients who respond positively to a vasodilator challenge have a more favorable prognosis (a 94% 5-year survival rate when they are treated with high-dose CCBs compared with 38% in those who are nonresponders).1
Common adverse effects of CCBs include severe peripheral edema and/or systemic hypotension, which may limit the effects of this medication. CCBs should be avoided in the absence of proven vasoreactivity (defined by the most recent criteria) and in patients with severe right-sided heart failure.
A diuretic is frequently helpful in patients with right-sided heart failure associated with volume overload, especially those with a right atrial pressure of 10 mm Hg or higher. However, special care must be used in adjusting this medication because these patients are preloaddependent and overdiuresis may result in hypotension.
Patients with right ventricular failure and venous stasis are at increased risk for pulmonary embolism. In addition, microscopic thrombosis has been found in patients with IPAH. Improved survival has been reported with oral anticoagulation therapy2; thus, this therapy is recommended.
Since hypoxemia is a potent pulmonary vasoconstrictor, supplemental oxygen should be used as needed to maintain oxygen saturations at 88% or higher. Digoxin may be used in patients with right-sided heart failure, but supportive data are limited to a single small study.3
Epoprostenol
A survival benefit has been demonstrated with intravenous epoprostenol. A multicenter, prospective, randomized trial compared the effects of epoprostenol plus conventional therapy with those of conventional therapy alone in patients who had IPAH with New York Heart Association (NYHA) class III or IV symptoms and found that survival was significantly greater for patients in the epoprostenol group at 12 weeks.4
Subsequently, compared with historical data, epoprostenol improved survival in patients with IPAH; 1-year and 3-year survival increased from 58.9% to 87.8% and 35.4% to 62.8%, respectively.5,6 Baseline characteristics associated with a worse outcome included a history of right-sided heart failure, NYHA functional class IV, 6-minute walk of less than 250 m and a right atrial pressure greater than 12 mm Hg.5,6 Survival of patients with IPAH depends on the severity at baseline and the response to therapy at 3 months or 1 year.5,6
Beyond epoprostenol's activity as a potent vasodilator, its mechanisms of benefit include a positive inotropic effect, a small degree of systemic vasodilation, and an antiplatelet effect.7 Because this agent acts as a central and peripheral vasodilator, it must be initiated very slowly to minimize adverse effects (nausea, vomiting, diarrhea, headaches, jaw pain, flushing) and the risk of systemic hypotension. The difficulty with epoprostenol is its administration by continuous intravenous infusion, the need for daily mixing, maintenance at a cool temperature (2°C to 8°C [35.6°F to 46.4°F]), and expense (up to $100,000 per year).
Treprostinil
The parental prostacyclin analogue treprostinil does not require mixing and is stable at room temperature, which improves convenience and ease of use. It can be administered as a continuous intravenous infusion, but subcutaneous infusion is preferable to reduce the risk of central venous catheter infection. Unfortunately, the subcutaneous form may be poorly tolerated because it has a high rate (80%) of infusion site discomfort.8
Endothelin receptor antagonists
Bosentan was the first in a new class of oral medications available for the treatment of IPAH. Plasma endothelin levels are increased in patients with PAH, and endothelin is released in increased amounts in the blood traversing the lung.9 Bosentan works by competitively binding to endothelin A (ETA) and endothelin B (ETB) receptor sites in the endothelium and vascular smooth muscle, preventing endothelin 1 from binding and triggering vasoconstriction.
When treated with bosentan for 16 weeks, patients who had IPAH or PH associated with connectivetissue disease had an improved 6-minute walk distance, improved Borg dyspnea index, improved World Health Organization (WHO) functional class, and longer time to clinical worsening.10 Early data analysis also suggests improved survival in patients treated with bosentan compared with historical controls.11,12 Bosentan appears to be as effective in elderly patients as in younger patients.13
Ambrisentan is a selective oral ETA antagonist that was recently approved as a daily agent for treatment of WHO class II-IV IPAH, PH associated with connective-tissue disease, and PH associated with HIV infection.14 Compared with patients who received placebo, patients with PAH who were treated with ambrisentan for 12 weeks demonstrated statistical improvement in 6-minute walk distance, WHO functional class, Short Form-36 Health Survey score, and time to clinical worsening.14
Endothelin binding to ETA and ETB receptors in vascular smooth muscle results in vasoconstriction, while binding of ETB receptors in endothelium causes vasodilation. Theoretically, the selective binding and inhibition of only the ETA receptors may improve the efficacy of ambrisentan compared with dualreceptor blockade. Once-daily dosing of ambrisentan is another possible advantage.14
Class effects of the endothelin receptor blockers are liver toxicity and teratogenicity. Therefore, effective birth control in women and monthly monitoring of liver function is required. No dosage adjustment is necessary in elderly patients; however, in the ambrisentan trial, the incidence of peripheral edema was greater in patients 65 years and older who received ambrisentan than in those who received placebo (29% vs 4%).14 This difference was not seen in patients younger than 65 years.
Other therapies
Sildenafil increases cyclic guanosine monophosphate (cGMP) levels by inhibiting phosphodiesterase type 5, an enzyme that hydrolyzes cGMP, thereby inducing vascular smooth muscle relaxation.15 Sildenafil has been shown to improve 6-minute walk distance, improve WHO functional class, and reduce the mean pulmonary artery pressure and pulmonary vascular resistance while increasing cardiac output in patients with WHO class II-IV PAH.16 The most common adverse effects included flushing, dyspepsia, and diarrhea.16
A study of aerosolized iloprost, a stable analogue of prostacyclin given by repeated daily inhalation, resulted in improvement of 6-minute walk distance, NYHA class, dyspnea, and quality of life.17 Surprisingly, adherence was higher within the treatment group than within the placebo group; however, effective dosing requires use between 7 and 9 times daily. Syncope was the most frequent adverse effect and occurred in both groups, although it was rated as more severe in the iloprost group.17
Further studies are examining the combination of medications that have different mechanisms of action. For example, the addition of inhaled iloprost to bosentan has been found to result in an increase in the 6-minute walk distance compared with bosentan alone.18
CONCLUSIONS
Dyspnea is a common symptom in the elderly that significantly affects quality of life and may predict a poor outcome. After other causes of dyspnea are eliminated and the diagnosis of PH is suspected, evaluation by echocardiography and confirmation by right heart catheterization should be done.
Treatment is guided by the cause of the PH. Progress in medical therapy for PAH has expanded remarkably in the past decade. Initial treatment should focus on general symptomatic management using diuretics, supplemental oxygen, and anticoagulants. Consider a trial of oral high-dose CCBs in the minority of patients who respond to a hemodynamic vasodilator challenge. Otherwise, patients with WHO functional class II-III IPAH or PH associated with connective-tissue disease should receive a trial of oral bosentan, ambrisentan, and/or sildenafil (Figure 2). Inhaled iloprost is an alternative or an additive agent.
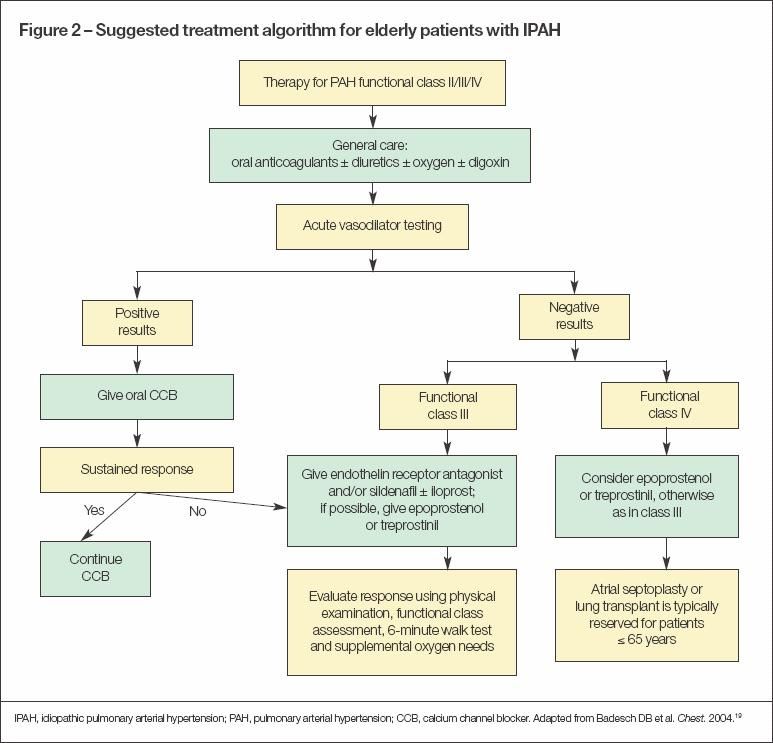
If patients fail to respond to these interventions or if they have WHO functional class IV PH, consider subcutaneous or intravenous treprostinil or epoprostenol.19 Treatment with these latter agents is much more complicated and may be difficult to initiate in the elderly patient.
Diastolic heart failure and pulmonary hypertension
Heart failure with normal ejection fraction (HFNEF) contributes to the largest number of cases of pulmonary hypertension (PH) in the elderly. Studies have shown that isolated HFNEF is present in 44% to 60% of patients with heart failure.20,21 In contrast to systolic dysfunction, HFNEF results from impaired myocardial relaxation resulting in decreased compliance and impairment of diastolic ventricular filling; the problem is not with ventricular contraction but rather with diastolic relaxation.
The most common causes of HFNEF are coronary artery disease, hypertension, aging, obesity, and aortic stenosis. The diagnosis is based on the presence of heart failure symptoms in the absence of depressed ejection fraction. An echocardiogram with Doppler studies can be useful in diagnosing diastolic dysfunction. The E/A ratio, in which E denotes the early peak mitral diastolic inflow velocity and A denotes the late diastolic peak mitral inflow velocity, can be useful.
Under normal conditions, E is greater than A and the E/A ratio is approximately 1.5. In early diastolic dysfunction, relaxation is impaired and there is vigorous atrial contraction, resulting in an E/A ratio of less than 0.75 (Figure 1). As the disease progresses, left ventricular (LV) compliance is reduced further, which increases early LV filling despite impaired relaxation, resulting in pseudonormalization of the E/A ratio to 1.5. In severe diastolic dysfunction, the E/A ratio is greater than 2 as a result of the LV filling occurring primarily in early diastole.22
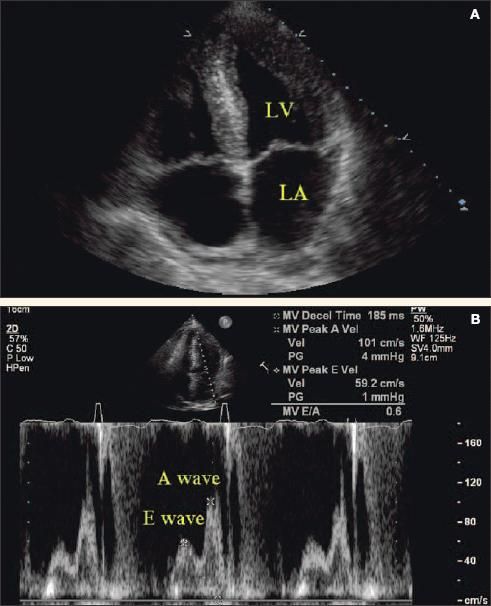
Figure 1 – Severe left ventricular hypertrophy and grade 1 diastolic heart failure are revealed by echocardiography and Doppler ultrasonography. The apical 4-chamber view shows a thickened left ventricular wall and enlarged left atrium (A). Doppler evaluation shows impaired early diastolic relaxation (E wave) and vigorous atrial contraction (A wave) resulting in an E/A ratio of less than 0.75, which signifies early reduced left ventricular compliance (B). A normal E/A ratio is greater than 1.5, since most of the diastolic filling occurs early in a compliant left ventricle with the atrial component contributing a smaller volume at a lower velocity. (LV, left ventricle; LA, left atrium.)
A pitfall of this technique is its dependence on changes in preload. The use of Doppler tissue imaging, an ultrasonographic modality that records systolic and diastolic velocities within the myocardium, can be used to measure the early diastolic velocity at the corner of the mitral annulus (Ea).23 The mitral E velocity corrected for the influence of myocardial relaxation (E/Ea ratio) may be a more accurate estimate of LV filling pressures.23
The most important means of treating diastolic dysfunction is to treat the underlying conditions, such as diabetes, hypertension, LV hypertrophy, coronary ischemia, anemia, and obesity. Given the similar pathophysiological abnormalities in systolic dysfunction and diastolic dysfunction, it has been assumed that proven therapy for systolic dysfunction will also be beneficial for diastolic dysfunction.
Despite the growing awareness of diastolic dysfunction, there have been few controlled clinical studies of drug therapies for patients with diastolic heart failure.21 The American College of Cardiology and the American Heart Association joint guidelines recommend blood pressure control, heart rate control, central blood volume reduction, the alleviation of myocardial ischemia, and restoration of sinus rhythm when atrial fibrillation is present.21
-Blockers and angiotensin-converting enzyme (ACE) inhibitors are associated with improved morbidity and perhaps improved survival in patients with diastolic heart failure.21,24-26 -Blockers reduce blood pressure and myocardial ischemia and antagonize the excessive adrenergic stimulation during heart failure. ACE inhibitors and angiotensin receptor blockers increase cardiac output and decrease LV filling pressure as a result of their vasodilatory effect. They also affect myocardial relaxation and compliance by inhibiting production or blocking angiotensin II receptors, thereby reducing interstitial collagen deposition and fibrosis.27 As in systolic dysfunction, diuretics are effective in optimizing intravascular volume and minimizing symptoms.
Calcium channel blockers (CCBs) and vasodilators (such as nitrates and hydralazine) have been shown to be beneficial in systolic heart failure, although they have not been studied in diastolic heart failure.28-30 CCBs theoretically improve diastolic function directly by decreasing cytoplasmic calcium concentration, causing myocardial relaxation. This reduces blood pressure and myocardial ischemia, which may promote regression of LV hypertrophy and slowing of the heart rate.31,32 The combination of hydralazine and isosorbide dinitrate was shown to increase survival in patients with systolic dysfunction in the Veterans Administration Heart Failure Trial (V-HeFT), although the study did not show significant survival benefit in patients with diastolic heart failure.28
Medications intended for patients with pulmonary arterial hypertension should not be used in this cohort of patients with pulmonary venous disease (World Health Organization class II disease). These expensive therapies have not been shown to be of benefit in this population and may result in pulmonary edema or hemodynamic compromise. This emphasizes the importance of obtaining the correct diagnosis of PH, which often mandates the performance of right heart catheterization.
References:
REFERENCES
1.
Rich S, Kaufmann E, Levy PS. The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension.
N Engl J Med.
1992;327:76-81.
2.
Fuster V, Steele PM, Edwards WD, et al. Primary pulmonary hypertension: natural history and the importance of thrombosis.
Circulation.
1984;70:580-587.
3.
Rich S, Seidlitz M, Dodin E, et al. The shortterm effects of digoxin in patients with right ventricular dysfunction from pulmonary hypertension.
Chest.
1998;114:787-792.
4.
Barst RJ, Rubin LJ, Long WA, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. The Primary Pulmonary Hypertension Study Group.
N Engl J Med.
1996;334:296-302.
5.
McLaughlin VV, Shillington A, Rich S. Survival in primary pulmonary hypertension: the impact of epoprostenol therapy.
Circulation.
2002;106:1477-1482.
6.
Sitbon O, Humbert M, Nunes H, et al. Longterm intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival.
J Am Coll Cardiol.
2002;40:780-788.
7.
Galiè N, Manes A, Branzi A. Medical therapy of pulmonary hypertension. The prostacyclins.
Clin Chest Med.
2001;22:529-537, x.
8.
Barst RJ, Galie N, Naeije R, et al. Long-term outcome in pulmonary arterial hypertension patients treated with subcutaneous treprostinil.
Eur Respir J.
2006;28:1195-1203.
9.
Giaid A, Yanagisawa M, Langleben D, et al. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension.
N Engl J Med.
1993;328:1732-1739.
10.
Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary arterial hypertension [published correction appears in N Engl J Med. 2002;346:1258].
N Engl J Med.
2002;346:896-903.
11.
McLaughlin VV, Sitbon O, Badesch DB, et al. Survival with first-line bosentan in patients with primary pulmonary hypertension [published correction appears in Eur Respir J. 2005;25:942].
Eur Respir J.
2005;25:244-249.
12.
Sitbon O, McLaughlin VV, Badesch DB, et al. Survival in patients with class III idiopathic pulmonary arterial hypertension treated with first line oral bosentan compared with an historical cohort of patients started on intravenous epoprostenol.
Thorax.
2005;60:1025-1030.
13.
Carrillo M, Cajigas H, Greenbaum A, Chan KM. Pulmonary hypertension in the elderly: demographics and outcomes following therapy with bosentan [abstract].
Chest.
2005;128:175S-176S.
14.
Galiè N, Olschewski H, Oudiz RJ, et al; Ambrisentan in Pulmonary Arterial Hypertension, Randomized, Double-Blind, Placebo-Controlled Multicenter, Efficacy Studies (ARIES) Group. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2.
Circulation.
2008;117:3010-3019.
15.
Prasad S, Wilkinson J, Gatzoulis MA. Sildenafil in primary pulmonary hypertension.
N Engl J Med.
2000;343:1342.
16.
Galie N, Ghofrani HA, Torbicki A, et al; Sildenafil Use in Pulmonary Arterial Hypertension (SUPER) Study Group. Sildenafil citrate therapy for pulmonary arterial hypertension [published correction appears in N Engl J Med. 2006;354:2400-2401].
N Engl J Med.
2005;353:2148-2157.
17.
Olschewski H, Simonneau G, Galiè N, et al; Aerosolized Iloprost Randomized Study. Inhaled iloprost for severe pulmonary hypertension.
N Engl J Med.
2002;347:322-329.
18.
McLaughlin VV, Oudiz RJ, Frost A, et al. Randomized study of adding inhaled iloprost to existing bosentan in pulmonary arterial hypertension.
Am J Respir Crit Care Med.
2006;174:1257-1263.
19.
Badesch DB, Abman SH, Ahearn GS, et al; American College of Chest Physicians. Medical therapy for pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines.
Chest.
2004;126(1 suppl):35S-62S.
20.
Bursi F, Weston SA, Redfield MM, et al. Systolic and diastolic heart failure in the community.
JAMA.
2006;296:2209-2216.
21.
Hunt SA; American College of Cardiology, American Heart Association Task Force on Practice Guidelines. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) [published correction appears in
J Am Coll Cardiol.
2006;47:1503-1505].
J Am Coll Cardiol.
2005;46:e1-e82.
22.
Oh JK, Hatle L, Tajik AJ, Little WC. Diastolic heart failure can be diagnosed by comprehensive two-dimensional and Doppler echocardiography.
J Am Coll Cardiol.
2006;47:500-506.
23.
Nagueh SF, Middleton KJ, Kopelen HA, et al. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures.
J Am Coll Cardiol.
1997;30:1527-1533.
24.
Chen HH, Lainchbury JG, Senni M, et al. Diastolic heart failure in the community: clinical profile, natural history, therapy, and impact of proposed diagnostic criteria.
J Card Fail.
2002;8:279-287.
25.
Yusuf S, Pfeffer MA, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial.
Lancet.
2003;362:777-781.
26.
Yip GW, Wang M, Wang T, et al. The Hong Kong diastolic heart failure study: a randomised controlled trial of diuretics, irbesartan and ramipril on quality of life, exercise capacity, left ventricular global and regional function in heart failure with a normal ejection fraction.
Heart.
2008;94:573-580.
27.
Mitsunami K, Inoue S, Maeda K, et al. Threemonth effects of candesartan cilexetil, an angiotensin II type 1 (AT1) receptor antagonist, on leftventricular mass and hemodynamics in patients with essential hypertension.
Cardiovasc Drugs Ther.
1998;12:469-474.
28.
Cohn JN, Johnson G. Heart failure with normal ejection fraction. The V-HeFT Study. Veterans Administration Cooperative Study Group.
Circulation.
1990;81(2 suppl):III48-53.
29.
Packer M, O’Connor CM, Ghali JK, et al. Effect of amlodipine on morbidity and mortality in severe chronic heart failure. Prospective Randomized Amlodipine Survival Evaluation Study Group.
N Engl J Med.
1996;335:1107-1114.
30.
Cohn JN, Ziesche S, Smith R, et al. Effect of the calcium antagonist felodipine as supplementary vasodilator therapy in patients with chronic heart failure treated with enalapril: V-HeFT III. Vasodilator-Heart Failure Trial (V-HeFT) Study Group.
Circulation.
1997;96:856-863.
31.
Little WC, Wesley-Farrington DJ, Hoyle J, et al. Effects of candesartan and verapamil on exercise tolerance in diastolic dysfunction.
J Cardiovasc Pharmacol.
2004;43:288-293.
32.
De Rosa ML, Giordano A, Della Guardia D, et al. Reversal of left ventricular hypertrophy following once daily administration of felodipine for two years to elderly subjects with isolated systolic hypertension.
Cardiology.
1999;92:39-44.
