- Clinical Technology
- Adult Immunization
- Hepatology
- Pediatric Immunization
- Screening
- Psychiatry
- Allergy
- Women's Health
- Cardiology
- Pediatrics
- Dermatology
- Endocrinology
- Pain Management
- Gastroenterology
- Infectious Disease
- Obesity Medicine
- Rheumatology
- Nephrology
- Neurology
- Pulmonology
Vaccination Recommendations for the 2008-2009 Influenza Season
Influenza develops in about 20% of the global population each year. In the United States, annual influenza epidemics typically occur between late December and early March. While influenza may affect persons of any age, infection rates are highest among children.
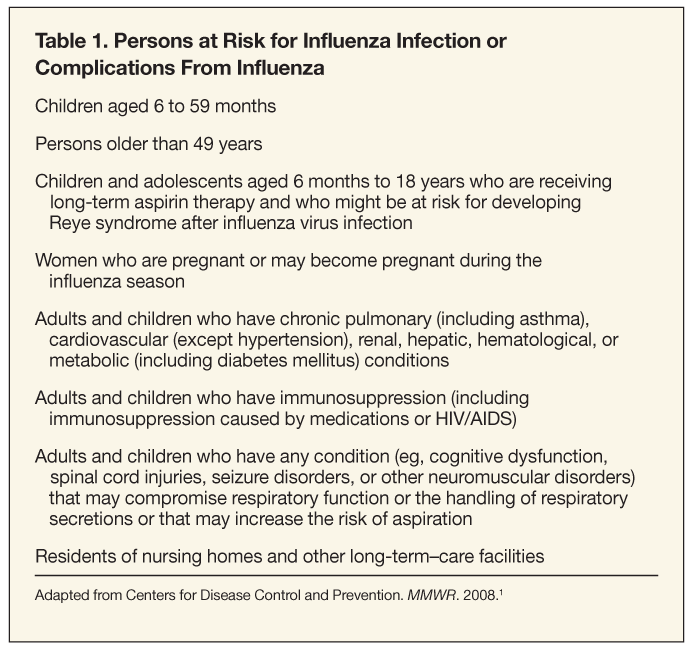
Each year, influenza develops in approximately 20% of the world’s population following infection with the influenza virus.1 In the United States, annual epidemics of influenza generally occur between late December and early March, although the influenza season extends from November through May.1 Influenza may develop in persons of any age, but rates are highest among children. Despite preventive efforts, influenza epidemics are responsible for significant morbidity and mortality every year. Influenza epidemics in the United States cause an average of 36,000 deaths, result in more than 225,000 hospitalizations and 31.4 million outpatient visits, and account for $16.3 billion in lost earnings annually.2-4 Serious illness, complications, and death attributable to influenza are highest among persons older than 65 years, children younger than 2 years, and persons with health conditions that place them at risk for complications from influenza (Table 1).3,5-7
Because of the enormous annual burden of influenza, managed care companies need to focus on the prevention of seasonal influenza. Annual influenza vaccination is the most effective method for preventing influenza virus infection and its complications. This article reviews influenza infection and discusses the recommendations of the CDC Advisory Committee on Immunization Practices on the use of influenza vaccines during the 2008-2009 influenza season.
Influenza Viruses
Influenza A and influenza B are the 2 types of influenza viruses that cause epidemics. There are 2 subtypes of influenza A that are based on surface antigens: hemagglutinin (HA) and neuraminidase (NA).1,6,8 Influenza B is separated into 2 distinct genetic lineages (Yamagata and Victoria) but is not categorized by subtypes. Since 1977, influenza A subtype H1N1, influenza A subtype H3N2, and influenza B have been circulating globally.1,6 All influenza vaccines are trivalent and contain these 3 strains of influenza virus. Each strain is defined by influenza type, geographic source, isolate number, year of isolation, HA subtype, and NA subtype (eg, A/Brisbane/10/2007/H3N2).1 New influenza virus variants are caused by frequent antigenic change (antigenic drift) resulting from point mutations that occur during viral replication.8 Each year, 1 or more vaccine strains are usually changed in response to results from global surveillance of circulating or emerging influenza virus strains.
The FDA and the World Health Organization recommended that the 2008-2009 influenza vaccine contain A/Brisbane/59/2007–like (H1N1) viruses, A/Brisbane/10/2007–like (H3N2) viruses, and B/Florida/4/2006–like viruses.1 Immunity to the surface antigens reduces the likelihood of infection.9 Increased levels of antibody caused by vaccinations lower the risk of illness caused by strains that are antigenically similar to those strains of the same type or subtype included in the vaccine.10
Transmission of influenza.
Influenza viruses are spread from person to person via large-particle respiratory droplet transmission (coughing or sneezing).11 The incubation period for influenza is 1 to 4 days, with an average of 2 days.8 Adults shed the virus from the day before symptoms appear to 5 to 10 days after illness onset.12,13 Children may shed the virus for more than 10 days after symptom onset, and immunocompromised persons may shed the virus for weeks to months.14-16
Influenza is characterized by the abrupt onset of fever, myalgias, headache, severe malaise, nonproductive cough, sore throat, and rhinitis. Otitis media, nausea, and vomiting may also develop in children with influenza. Symptoms of influenza typically resolve within 3 to 7 days; however, cough and malaise may persist for more than 2 weeks.1 Influenza-related complications requiring urgent care can result from the direct effects of influenza, complications associated with age or pregnancy, or complications related to underlying medical conditions.6
Prevention of influenza.
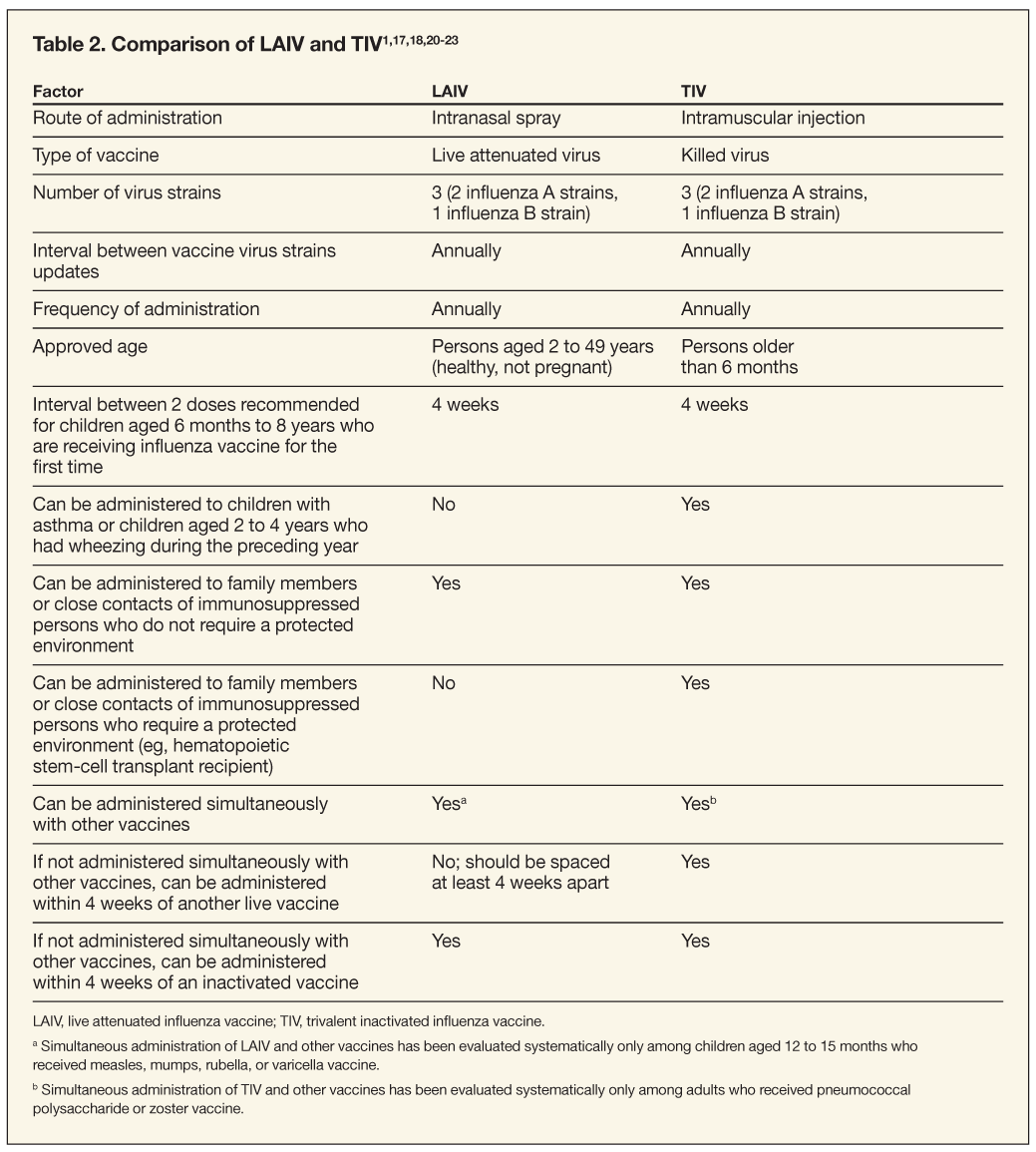
Annual vaccination is the most effective strategy for preventing influenza. Currently, there are 2 types of vaccines: trivalent inactivated influenza vaccine (TIV) and live attenuated influenza vaccine (LAIV) (Table 2). The clinical outcomes of influenza vaccines depend on the age and immune status of the patient, the degree of similarity between the virus strains in the vaccine and those in circulation, and the end point measured in trials. TIV and LAIV contain strains of influenza viruses that are antigenically equivalent to the annually recommended strains: one influenza A H3N2 virus, one influenza A H1N1 virus, and one influenza B virus. All 3 vaccine strains were changed for the 2008-2009 influenza season.1 Although both types of vaccines are effective, they differ in several respects (see Table 2). The biggest differences are the route of administration and whether the vaccine should be given to children with asthma or children aged 2 to 4 years with wheezing during the preceding year.
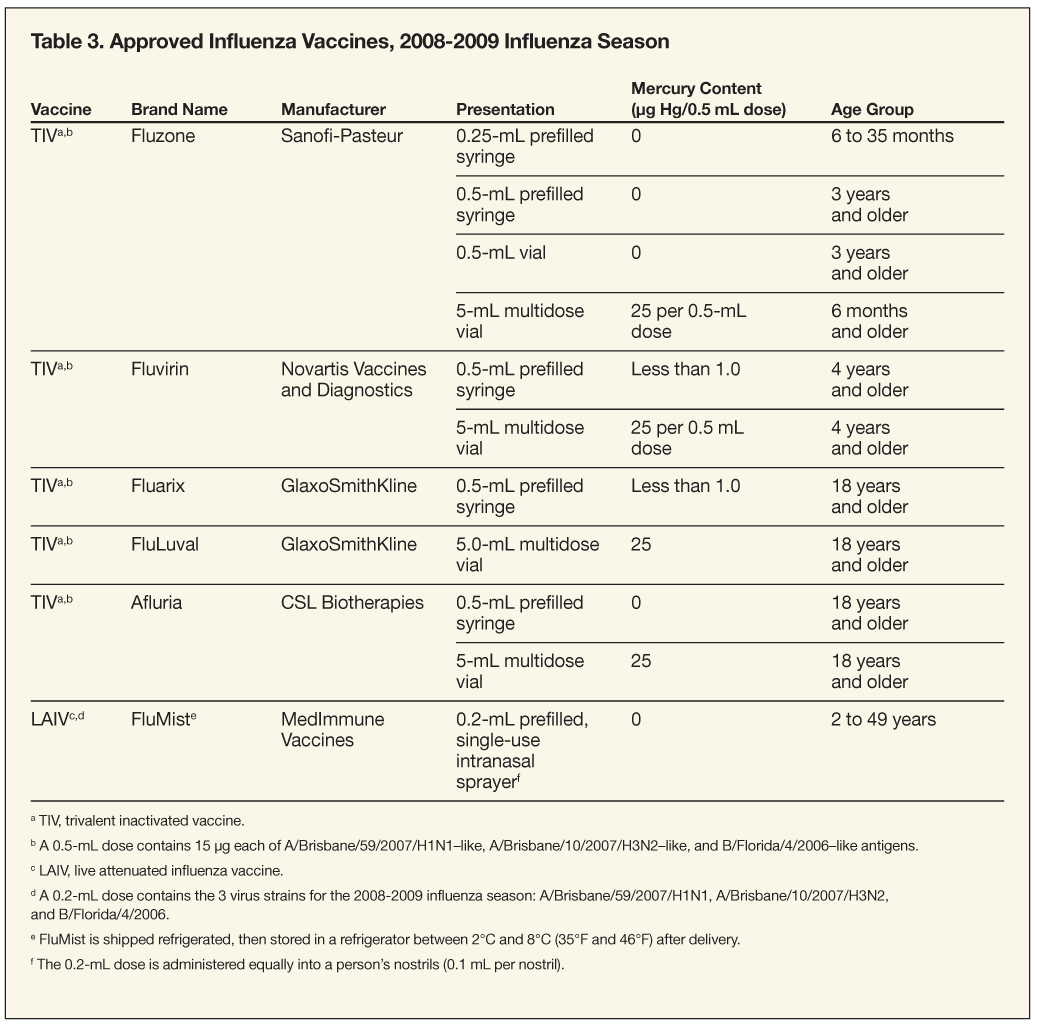
TIV. Because TIV (Fluzone, Fluvirin, Fluarix, FluLuval, Afluria) contains killed or split viruses, it cannot cause influenza. TIV is administered intramuscularly in the deltoid muscle in adults and older children.17-20 Infants and young children should be vaccinated in the anterolateral aspect of the thigh. TIV is available both in multidose vials that contain the vaccine preservative thimerosal and as preservative-free single-dose preparations.17,18 Fluzone is supplied in 4 formulations: pediatric-dose, prefilled syringes that contain 0.25 mL of vaccine, without preservative; prefilled syringes that contain 0.5 mL of vaccine, without preservative; single-dose vials that contain 0.5 mL of vaccine, without preservative; and multidose vials that contain 5 mL of vaccine plus thimerosal.17 Fluvirin is supplied in 2 formulations: prefilled syringes that contain 0.5 mL of vaccine, without preservative, and multidose vials that contain 5 mL of vaccine plus thimerosal.18 Fluarix is supplied as prefilled syringes that contain 0.5 mL of vaccine and a small amount of thimerosal.20 FluLuval is supplied as multidose vials that contain 5 mL of vaccine as well as thimerosal.21 Afluria is supplied in 2 formulations: prefilled syringes that contain 0.5 mL of vaccine without preservative and multidose vials that contain 5 mL of vaccine plus thimerosal.22 TIV should be stored between 2°C and 8°C (35°F and 46°F) and should not be frozen. TIV that has been frozen should be discarded.
LAIV. LAIV (FluMist) contains live attenuated viruses that can cause mild signs or symptoms of influenza (runny nose, nasal congestion, fever, sore throat). LAIV does not cause systemic symptoms of influenza because the live attenuated viruses are able to replicate efficiently only at temperatures present in the nasal mucosa. LAIV is administered intranasally by sprayer.
Each dose of LAIV contains the same 3 vaccine antigens used in TIV. LAIV is supplied in a prefilled, single-use intranasal sprayer that contains 0.2 mL of vaccine.23 Approximately 0.1 mL (half of the sprayer contents) is sprayed into the first nostril while the patient is in an upright position. Then an attached dose-divider clip is removed from the sprayer for the second half of the dose to be administered into the other nostril. LAIV should be stored at 2°C to 8°C (35°F to 46°F) and should not be frozen.
Safety. TIV is recommended for use in persons 6 months and older, including persons who are healthy as well as those with chronic conditions (Table 3). LAIV is indicated only for healthy persons aged 2 to 49 years who are not pregnant. The safety of annual vaccination of TIV and LAIV in children and adolescents is supported by several studies. Data on adverse events among children after influenza vaccination are available from the Vaccine Adverse Event Reporting System (VAERS).
TIV in children. A population analysis of TIV in 251,600 children younger than 18 years, including 8476 children aged 6 to 23 months, did not find a significant increase in adverse events during the 2 weeks after vaccination compared with adverse events reported during 2 control periods that lasted 3 or 4 weeks.24 Another analysis demonstrated that there were no statistically significant increases in medical outcomes among 45,356 children aged 6 to 23 months.25 Vaccinated children were less likely to report acute respiratory illness, otitis media, and asthma. Older analyses have found that fever, malaise, myalgias, and other systemic symptoms can develop in children after vaccination with TIV.26,27 The symptoms often develop in children with no previous exposure to the influenza virus antigens in TIV.
LAIV in children. The most common adverse reactions of children who received LAIV are nasal congestion, runny nose, and fever. LAIV should not be administered to children younger than 24 months. Clinical trials have demonstrated an increased risk of postvaccination wheezing in this age group. LAIV also should not be administered to any person with asthma and to children younger than 5 years who have recurrent wheezing because of the increased risk of postvaccination wheezing.28-33
TIV in adults. Among adults who received TIV, vaccination-site soreness was the adverse effect most frequently reported in placebo-controlled trials and lasted less than 2 days.34,35 Local reactions were mild and rarely affected the persons’ ability to conduct daily activities. Administration of TIV to older persons and healthy adults was not associated with higher rates of systemic symptoms (fever, malaise, myalgias, headache) when compared with placebo.34-37
TIV is a use-in-pregnancy Category C medication, an FDA rating that indicates either that animal studies have shown an adverse effect or no animal studies have been conducted, and that in both situations, there are no adequate and well-controlled studies in pregnant women.38 However, available data show that TIV does not cause fetal harm when administered to pregnant women. Between 2000 and 2003, an estimated 2 million pregnant women were vaccinated, and only 20 adverse events were reported.39 There were 9 injection site reactions and 8 systemic reactions (fever, headache, myalgias).
LAIV in adults. Among adults, runny nose, nasal congestion, headache, and sore throat have been reported more often among persons given LAIV than among those who received placebo.40,41 LAIV was well tolerated among adults older than 65 years with chronic conditions.42 The safety of LAIV has not been established in persons with underlying medical conditions that increase their risk of influenza complications (eg, reactive airway disease, renal dysfunction).
As with TIV use, animal reproduction studies have not been conducted with LAIV administration. It is not known whether LAIV can cause fetal harm when administered to pregnant women.
Adverse reactions Allergic reactions. Allergic reactions rarely occur after influenza vaccination. Immediate reactions, such as hives, angioedema, allergic asthma, and systemic anaphylaxis, most likely result from hypersensitivity to certain vaccine components. Most allergic reactions are probably caused by residual egg protein. Although both TIV and LAIV contain a limited quantity of egg protein, it can induce immediate hypersensitivity reactions among persons who have a severe egg allergy.43
Guillain-Barr syndrome. This is a rare condition (10 to 20 cases per million adults) with multiple causes.1 No evidence exists for a consistent association between vaccines prepared from influenza viruses and Guillain-Barr syndrome (GBS). (The only exception is the 1976 swine influenza vaccine, which was associated with an increased frequency of GBS, estimated to be 1 additional case of GBS per 100,000 persons vaccinated. No studies on the use of other influenza vaccines have demonstrated a substantial increase in GBS, according to VAERS.44-47)
In 3 of 4 influenza seasons analyzed between 1977 and 1991, the overall relative risk estimates of GBS after influenza vaccination were not statistically significant. However, in the 1992-1993 and 1993-1994 influenza seasons, the relative risk for GBS was 1.7 (95% confidence interval, 1.0 - 2.8; P = .04) during the 6 weeks after vaccination.44 This represents 1 additional case of GBS per million persons vaccinated. Data from VAERS reports showed decreases in GBS among all age groups over time but increases in other adverse effects (eg, swelling, fever, headache, malaise) after influenza vaccination.34 The benefits of influenza vaccination in preventing serious illness outweigh these estimates of risk of vaccine-associated GBS.
Hypersensitivity reaction to thimerosal. Multidose vials of TIV contain thimerosal to reduce the likelihood of bacterial contamination. Adverse reactions associated with this preservative, which contains mercury, are rare and consist of local hypersensitivity reactions. No evidence has demonstrated harm from exposure to vaccines that contain thimerosal. Thimerosal has not been found to cause neurodevelopmental disorders or adverse events among children of women who received the vaccine during pregnancy.48-52
However, public concern about mercury exposure in vaccines can be a barrier to achieving goal vaccination rates. As a result, the US Public Health Service recommended eliminating or reducing the amount of thimerosal in vaccines. Since mid-2001, vaccines recommended for children younger than 6 months have been manufactured either without thimerosal or with greatly reduced amounts of the preservative (see Table 3). LAIV and many of the single-dose preparations of TIV do not contain thimerosal. The benefits of influenza vaccination for all persons for whom vaccination is recommended, including pregnant women and young children, outweigh concerns about the theoretical risk of thimerosal exposure through vaccination.
Comparison of Efficacy of TIV and LAIV
TIV and LAIV have been demonstrated to be effective in children and adults, but data directly comparing the 2 vaccines are limited. Although most comparative data on TIV and LAIV did not show statistical differences in outcomes in preventing laboratory-documented influenza or influenza-like illness, a few analyses had different outcomes. A randomized clinical trial of children aged 6 to 71 months during the 2004-2005 influenza season found a 54.9% reduction of culture-confirmed influenza among children who received LAIV compared with those who received TIV.33 The researchers also found that LAIV efficacy was higher than that of TIV against antigenically drifted viruses as well as well-matched viruses. Efficacy against antigenically drifted viruses is important because influenza vaccines are not always matched with circulating strains-a result of the continuous antigenic evolution of wild-type influenza. Vaccine mismatch has occurred in 6 of the past 12 years. A nonrandomized, community-based trial conducted among persons aged 5 to 18 years indicated that LAIV was effective against antigenically drifted H3N2 compared with TIV.53
Although LAIV is not approved for use in persons with risk factors for influenza complications (eg, asthma) or children younger than 2 years, studies have been conducted that have compared the efficacy of LAIV with that of TIV in these groups of patients. Among children and adolescents aged 6 to 17 years with asthma, there were 34.7% fewer cases of influenza in those who received LAIV than in those who received TIV.54 Compared with TIV efficacy among children aged 6 to 71 months with recurrent respiratory tract infections, LAIV efficacy was 52.7%.55
Recommendations for Using TIV and LAIV During the 2008-2009 Influenza Season
TIV and LAIV can be used to reduce the risk of influenza virus infection and its complications. Vaccination should be administered to any person who wants to reduce the likelihood of developing influenza and/or transmitting influenza to others. However, emphasis should be placed on vaccinating persons at higher risk for influenza infection and complications: children aged 6 months to 18 years and persons older than 49 years. About 83% of the US population is included in target groups; however, less than 40% of the population received an influenza vaccination during the 2007-2008 influenza season. Healthy persons aged 2 to 49 years who are not pregnant can choose to receive either vaccine. Some TIV formulations are FDA-approved for use in children as young as 6 months (see Table 3). TIV is also recommended for use in persons with high-risk conditions (see Table 1).1
Children aged 6 months to 18 years. Beginning with the 2008-2009 influenza season, annual vaccination for all children aged 6 months to 18 years is recommended. Children aged 6 to 59 months and adolescents at high risk for influenza complications (eg, asthma) should continue to be a focus of vaccination efforts. Annual vaccination of all children aged 5 to 18 years should have begun in September 2008 for the current influenza season and should begin each year as soon as vaccine is available. Annual vaccination of all children aged 5 to 18 years should begin no later than the 2009-2010 influenza season. Healthy children aged 2 to 18 years can receive either LAIV or TIV. Children aged 6 to 23 months, those aged 2 to 4 years who show signs of reactive airway disease, and those who have medical conditions that put them at higher risk for influenza complications should receive TIV. All children aged 6 months to 8 years who have not been vaccinated previously with at least 1 dose of LAIV or TIV should receive 2 doses of age-appropriate vaccine at least 4 weeks apart in the same influenza season.1
Persons who live with or care for persons at high risk for influenza. To prevent transmission to persons at high risk for influenza (see Table 1), vaccination with TIV or LAIV is recommended.1 Vaccination efforts should focus on the following persons:
• Health care personnel (HCP).
• Healthy household contacts (including children) and caregivers of children younger than 59 months and adults older than 49 years.
• Healthy household contacts and caregivers of persons with medical conditions that put them at higher risk for influenza complications.

Health care personnel. All HCP should be vaccinated annually against influenza (Table 4).1 Facilities that employ HCP should provide vaccine to workers by using approaches that have been demonstrated to increase vaccination rates. Health care facilities should consider employee vaccination rates as a measure of a patient safety quality program and consider obtaining signed refusal forms from personnel who decline influenza vaccination. Healthy HCP and persons aged 2 to 49 years who are contacts of persons at high risk for influenza and who are not contacts of severely immunocompromised persons should receive either LAIV or TIV. All other HCP should receive TIV.
Close contacts of immunocompromised persons. Close contacts of immunocompromised persons, including HCP, should be vaccinated to reduce the risk of influenza transmission.1 TIV is preferred for vaccinations for household members, HCP, and other persons who have close contact with severely immunocompromised persons (eg, persons who have had a hematopoietic stem cell transplant) during periods in which such persons require care in a protective environment (typically defined as a specialized patient care area with a positive airflow relative to that of the corridor, high-efficiency particulate air filtration, and frequent air changes).56,57
The use of LAIV among HCP or other close contacts of severely immunocompromised persons is avoided because of the theoretical risk that a live attenuated vaccine virus could be transmitted to these patients. As a precautionary measure, HCP who receive LAIV should avoid providing care for severely immunocompromised persons for 7 days after vaccination. Hospital visitors who have received LAIV should avoid contact with such persons in protected environments for 7 days after vaccination but should not be restricted from visiting less severely immunocompromised patients.

Pregnant and nursing women. All women who are pregnant or may become pregnant during the influenza season should be vaccinated with TIV.1,58 LAIV is not recommended for use by pregnant women. However, pregnant women do not need to avoid contact with persons who have recently received LAIV. Vaccination is recommended for all persons, including nursing mothers, who are in close contact with children younger than 59 months. This is because infants and young children are at high risk for influenza complications and are more likely to require medical care or hospitalization if infected. Women who are nursing can receive either TIV or LAIV unless contraindicated because of other medical conditions (Table 5).1
General population. Vaccination is recommended for persons who want to lower the likelihood of influenza developing and/or transmitting it if they become infected.1 Healthy persons aged 2 to 49 years who are not pregnant may be vaccinated with LAIV. All persons 6 months and older may receive TIV, regardless of past medical history or pregnancy status. When vaccinating children aged 6 to 35 months with TIV, health care providers should administer TIV that has been approved by the FDA for this age group (see Table 3). Persons who provide community services should be vaccinated in order to minimize disruption of essential activities during the influenza season. Students and other persons who live or work in institutional settings should be encouraged to receive the influenza vaccine.
Conclusion
Annual epidemics of influenza have a significant impact on clinical and economic outcomes in the United States. Influenza may be prevented by annual vaccination with LAIV or TIV. There are new vaccination guidelines for the 2008-2009 influenza season, and the influenza vaccine has 3 new strains. Managed care organizations should be alert to these recommendations and focus on vaccinating target groups, such as children aged 6 months to 18 years and persons older than 49 years, and any person who wants to reduce the likelihood of becoming ill with influenza and/or transmitting influenza if he or she becomes infected.
References:
References
1. Centers for Disease Control and Prevention. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 2008;57:1-60. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr57e717a1.htm. Accessed December 16, 2008.
2. Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179-186.
3. Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333-1340.
4. Molinari NA, Ortega-Sanchez IR, Messonnier ML, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25:5086-5096.
5. Cho BH, Kolasa MS, Messonnier ML. Influenza vaccination coverage rate among high-risk children during the 2002-2003 influenza season. Am J Infect Control. 2008;36:582-587.
6. Beigel JH. Influenza. Crit Care Med. 2008;36:2660-2666.
7. Schanzer DL, Langley JM, Tam TW. Co-morbidities associated with influenza-attributed mortality, 1994-2000, Canada. Vaccine. 2008;26:4697-4703.
8. Cox NJ, Subbarao K. Influenza. Lancet. 1999;354: 1277-1282.
9. Clements ML, Betts RF, Tierney EL, Murphy BR. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild-type virus. J Clin Microbiol. 1986;24:157-160.
10. Couch RB, Kasel JA. Immunity to influenza in man. Annu Rev Microbiol. 1983;37:529-549.
11. Brankston G, Gitterman L, Hirji Z, et al. Transmission of influenza A in human beings. Lancet Infect Dis. 2007;7:257-265.
12. Leekha S, Zitterkopf NL, Espy MJ, et al. Duration of influenza A virus shedding in hospitalized patients and implications for infection control. Infect Control Hosp Epidemiol. 2007;28:1071-1076.
13. Treanor J. Influenza virus. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005:2060-2085.
14. Hall CB, Douglas RG Jr. Nosocomial influenza infection as a cause of intercurrent fevers in neonates. Pediatrics. 1975;55:673-677.
15. Englund JA, Champlin RE, Wyde PR, et al. Common emergence of amantadine- and rimantadine-resistant influenza A viruses in symptomatic immunocompromised adults. Clin Infect Dis. 1998;26:1418-1424.
16. Boivin G, Goyette N, Bernatchez H. Prolonged excretion of amantadine-resistant influenza a virus quasi species after cessation of antiviral therapy in an immunocompromised patient. Clin Infect Dis. 2002;34:E23-E25.
17. Fluzone [prescribing information]. Swiftwater, PA: Sanofi-Pasteur; 2006.
18. Fluvirin [prescribing information]. Emeryville, CA: Novartis Vaccines and Diagnostics; 2006.
19. Poland GA, Borrud A, Jacobson RM, et al. Determination of deltoid fat pad thickness. Implications for needle length in adult immunization. JAMA. 1997;277:1709-1711.
20. Fluarix [prescribing information]. Research Triangle Park, NC: GlaxoSmithKline; 2008-2009.
21. FluLuval [prescribing information]. Research Triangle Park, NC: GlaxoSmithKline; 2008-2009.
22. Afluria [prescribing information]. King of Prussia, PA: CSL Biotherapies; 2008-2009.
23. FluMist [prescribing information]. Gaithersburg, MD: MedImmune Vaccines; 2007.
24. France EK, Glanz JM, Xu S, et al. Safety of the trivalent inactivated influenza vaccine among children: a population-based study. Arch Pediatr Adolesc Med. 2004;158:1031-1036.
25. Hambidge SJ, Glanz JM, France EK, et al. Safety of trivalent inactivated influenza vaccine in children 6 to 23 months old. JAMA. 2006;296:1990-1997.
26. Neuzil KM, Dupont WD, Wright PF, Edwards KM. Efficacy of inactivated and cold-adapted vaccines against influenza A infection, 1985 to 1990: the pediatric experience. Pediatr Infect Dis J. 2001;20:733-740.
27. Barry DW, Mayner RE, Hochstein HD, et al. Comparative trial of influenza vaccines, II: adverse reactions in children and adults. Am J Epidemiol. 1976;104:47-59.
28. Zangwill KM, Droge J, Mendelman P, et al. Prospective, randomized, placebo-controlled evaluation of the safety and immunogenicity of three lots of intranasal trivalent influenza vaccine among young children. Pediatr Infect Dis J. 2001;20:740-746.
29. Nolan T, Lee MS, Cordova JM, et al. Safety and immunogenicity of a live-attenuated influenza vaccine blended and filled at two manufacturing facilities. Vaccine. 2003;21:1224-1231.
30. Vesikari T, Fleming DM, Aristegui JF, et al. Safety, efficacy, and effectiveness of cold-adapted influenza vaccine-trivalent against community-acquired, culture-confirmed influenza in young children attending day care. Pediatrics. 2006;118: 2298-2312.
31. Redding G, Walker RE, Hessel C, et al. Safety and tolerability of cold-adapted influenza virus vaccine in children and adolescents with asthma. Pediatr Infect Dis J. 2002;21:44-48.
32. Bergen R, Black S, Shinefield H, et al. Safety of cold-adapted live attenuated influenza vaccine in a large cohort of children and adolescents. Pediatr Infect Dis J. 2004;23;138-144.
33. Belshe RB, Edwards KM, Vesikari T, et al. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med. 2007; 356:685-696.
34. Govaert TM, Dinant GJ, Aretz K, et al. Adverse reactions to influenza vaccine in elderly people: randomised double blind placebo controlled trial. BMJ. 1993;307:988-990.
35. Margolis KL, Nichol KL, Poland GA, Pluhar RE. Frequency of adverse reactions to influenza vaccine in the elderly. A randomized, placebo-controlled trial. JAMA. 1990;264:1139-1141.
36. Cates CJ, Jefferson TO, Rowe BH. Vaccines for preventing influenza in people with asthma. Cochrane Database Syst Rev. 2008;(2):CD000364.
37. Nichol KL, Margolis KL, Lind A, et al. Side effects associated with influenza vaccination in healthy working adults. A randomized, placebo-controlled trial. Arch Intern Med.1996;156:1546-1550.
38. Food and Drug Administration. Current categories for drug use in pregnancy; 2001. www.fda.gov/fdac/features/2001/301_preg.html#categories. Accessed December 16, 2008.
39. Pool V, Iskander J. Safety of influenza vaccination during pregnancy. Am J Obstet Gynecol. 2006; 194:1200.
40. Jackson LA, Holmes SJ, Mendelman PM, et al. Safety of a trivalent live attenuated intranasal influenza vaccine, FluMist, administered in addition to parenteral trivalent inactivated influenza vaccine to seniors with chronic medical conditions. Vaccine. 1999;17:1905-1909.
41. Nichol KL, Mendelman PM, Mallon KP, et al.Effectiveness of live, attenuated intranasal influenza virus vaccine in healthy, working adults: a randomized controlled trial. JAMA. 1999;282: 137-144.
42. Izurieta HS, Haber P, Wise RP, et al. Adverse events reported following live, cold-adapted, intranasal influenza vaccine. JAMA. 2005;294:2720-2725.
43. Zeiger RS. Current issues with influenza vaccination in egg allergy. J Allergy Clin Immunol. 2002;110:834-840.
44. Lasky T, Terracciano GJ, Magder L, et al. The Guillain-Barré syndrome and the 1992-1993 and the 1993-1994 influenza vaccines. N Engl J Med. 1998;339:1797-1802.
45. Haber P, DeStefano F, Angulo FJ, et al. Guillain-Barré syndrome following influenza vaccination. JAMA. 2004;292:2478-2481.
46. Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, et al. Guillain-Barre syndrome following vaccination in the National Influenza Immunization Program, United States, 1976-1977. Am J Epidemiol. 1979;110:105-123.
47. Juurlink DN, Stukel TA, Kwong J, et al. Guillain-Barré syndrome after influenza vaccination in adults: a population-based study. Arch Intern Med. 2006;166:2217-2221.
48. Centers for Disease Control and Prevention. Summary of the joint statement on thimerosal in vaccines.MMWR. 2000;49:622-631. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm4927a5.htm. Accessed December 16, 2008.
49. Verstraeten T, Davis RL, DeStefano F, et al. Safety of thimerosal-containing vaccines: a two-phased study of computerized health maintenance organization databases. Pediatrics. 2003; 112:1039-1048.
50. Pichichero ME, Cernichiari E, Lopreiato J, Treanor J. Mercury concentrations and metabolism in infants receiving vaccines containing thiomersal: a descriptive study. Lancet. 2002;360:1737-1741.
51. Pichichero ME, Gentile A, Giglio N, et al. Mercury levels in newborns and infants after receipt of thimerosal-containing vaccines. Pediatrics. 2008;121:e208-e214.
52. Thompson WW, Price C, Goodson B, et al. Early thimerosal exposure and neuropsychological outcomes at 7 to 10 years. N Engl J Med. 2007; 357:1281-1292.
53. Piedra PA, Gaglani MJ, Riggs M, et al. Live attenuated influenza vaccine, trivalent, is safe in healthy children 18 months to 4 years, 5 to 9 years, and 10 to 18 years of age in a community-based, nonrandomized, open-label trial. Pediatrics. 2005;116:e397-e407.
54. Fleming DM, Crovari P, Wahn U, et al. Comparison of the efficacy and safety of live attenuated cold-adapted influenza vaccine, trivalent, with trivalent inactivated influenza virus vaccine in children and adolescents with asthma. Pediatr Infect Dis J. 2006;25:860-869.
55. Ashkenazi S, Vertruyen A, ArÃstegui J, et al; CAIV-T Study Group. Superior relative efficacy of live attenuated influenza vaccine compared with inactivated influenza vaccine in young children with recurrent respiratory tract infections. Pediatr Infect Dis J. 2006;25:870-879.
56. Centers for Disease Control and Prevention. Influenza vaccination of health-care personnel: recommendations of the Healthcare Infection Control Practices Advisory Committee (HICPAC) and the Advisory Committee on Immunization Practices (ACIP). MMWR. 2006; 55 (RR02):1-16. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5502a1.htm. Accessed December 16, 2008.
57. Centers for Disease Control and Prevention. Guidelines for environmental infection control in health-care facilities: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR. 2003;52(RR10):1-42. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5210a1.htm. Accessed December 16, 2008.
58. Centers for Disease Control and Prevention. Recommended adult immunization schedule-United States, October 2006-September 2007. MMWR. 2006;55(40):Q1-Q4. http://www.cdc.gov/mmwr/pdf/wk/mm5540-Immunization.pdf. Accessed December 30, 2008.
