- Clinical Technology
- Adult Immunization
- Hepatology
- Pediatric Immunization
- Screening
- Psychiatry
- Allergy
- Women's Health
- Cardiology
- Pediatrics
- Dermatology
- Endocrinology
- Pain Management
- Gastroenterology
- Infectious Disease
- Obesity Medicine
- Rheumatology
- Nephrology
- Neurology
- Pulmonology
Tirzepatide Real-World Benefit Found Greater for CV, Renal Outcomes vs GLP-1 RAs in Adults with T2D
In a head-to-head real world study, tirzepatide compared with semaglutide resulted in 42%, 20%, and 46% reduced risks for all-cause mortality and major adverse CV and renal events, respectively.
©MKPhoto/stock.adobe.com
Treatment with the dual incretin receptor agonist tirzepatide (Mounjaro; Lilly) of adults with type 2 diabetes (T2D) was associated with a lower risk of all-cause mortality and adverse cardiovascular (CV) and renal events compared with glucagon-like peptide-1 receptor agonists (GLP1-RA) in a real-world study of more than 140 000 participants.
The head-to-head investigation is the first to compare the 2 incretin-based medications and tapped comprehensive medical information from the US Collaborative Network in TriNetX, which includes electronic health record data for more than 100 million individuals attending 62 US health care organizations.
The findings, reported in JAMA Network Open Diabetes and Endocrinology, “advocate for the integration of tirzepatide into therapeutic strategies for managing type 2 diabetes and highlight its potential to enhance clinical practice,” Vin-cent Wu, MD, PhD, attending physician in the department of internal medicine at National Taiwan University Hospital, and colleagues wrote.
Specifically, Wu and colleagues found that over an average follow-up period of 10.5 months study participants treated with tirzepatide vs GLP1-RAs had a statistically significant:
- 42% reduced relative risk for all-cause mortality
- 46% lower risk of major adverse kidney events (MAKE)
- 20% lower risk of major adverse cardiovascular events (MACE)
- 24% lower risk of a composite MACE and all-cause mortality outcome
- 22% lower risk of acute kidney injury
Further analysis supported an “unlikely significant influence from unmeasured confounders,” researchers wrote.
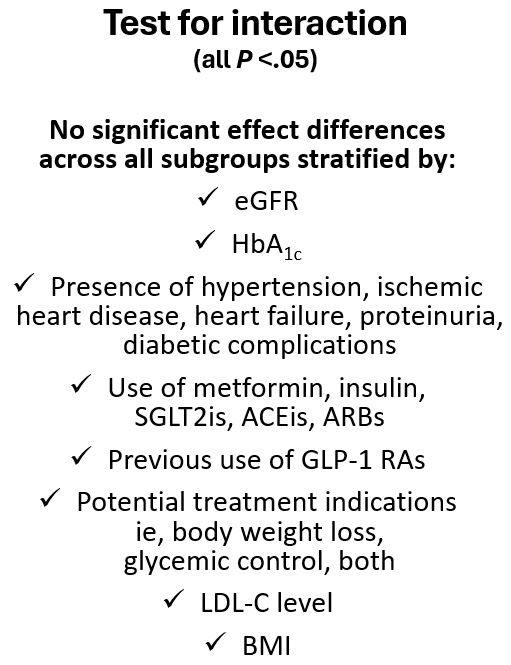
The research team also reported greater reductions in HbA1c (treatment difference -0.34 percentage points), and body weight (treatment difference -2.9 kg) for tirzepatide-treated participants compared with participants receiving GLP1-RA treatment over a period of more than 20 months. When they assessed timing and stabilization of intervention effect on these outcomes, Wu et al observed the most significant reduction in HbA1c during the first 4 months of follow-up with decreases stabilizing after approximately 8 months. Loss of body weight continued through 16 to 20 months, according to the study. Findings of sensitivity analyses are noted in the Figure at left.
The association of tirzepatide with less all-cause mortality and MACEs may be attributable to the medication' efficacy in improving the CV profile, including blood pressure, LDL, triglycerides, HbA1c, cardiovascular risk biomarkers, and body weight in patients with obesity.2-4
Study rationale
There are numerous studies supporting the benefits of GLP1-RAs on kidney and CV outcomes and evidence for the superiority of tirzepatide, a dual GLP-1 and glucose-dependent insulinotropic polypeptide RA, over semaglutide for reducing HbA1c and weight and over insulin glargine for ameliorating eGFR decline and reducing UACR in adults with T2D, according to Wu et al. They also cite research that demonstrates a more favorable profile for tirzepatide vs semaglutide on reducing atherogenic lipoproteins and blood pressure. Missing from the literature, however, is evidence of whether the salutary metabolic effects of tirzepatide lead to benefits in survival and renal or CV outcomes and also any head-to-head comparison of the mono- and dual-incretin mimetics’ effects on mortality and MACE and MAKE in adults with T2D. The data from their study may help begin to build that evidence foundation.
Led by Wu, the investigators searched the TriNetX database for adults aged 18 years and older with type 2 diabetes who had been prescribed tirzepatide or a GLP1-RA between June 1, 2022, and June 30, 2023. The study protocol called for exclusion of any potential participant with stage 5 kidney disease, end stage kidney disease or receiving dialysis at baseline.
Of the final 140,308 participants (mean age 56,7 years, 55.3% women) 14,384 were treated with tirzepatide and 125,474 with GLP1 RA. All participants were followed for a maximum of 21 months or until data analysis on May 2, 2024. After a median on 10.5 months, there were 95 deaths in the in the tirzepatide group and 166 in the GLP1 RA group, according to the results.
Missing from the literature, however, is evidence of whether the salutary metabolic effects of tirzepatide lead to benefits in survival and renal or CV outcomes and also any head-to-head comparison of the mono- and dual-incretin mimetics’ effects on mortality and MACE and MAKE in adults with T2D. The data from their study may help begin to build that evidence foundation.
The investigators' final analyses found that tirzepatide treatment was associated with lower hazards of all-cause mortality (adjusted hazard ratio [AHR], 0.58; 95% CI, 0.45-0.75), MACEs (aHR, 0.80; 95% CI, 0.71-0.91), the composite of MACEs and all-cause mortality (aHR, 0.76; 95% CI, 0.68-0.84), kidney events (aHR, 0.52; 95% CI, 0.37-0.73), acute kidney injury (aHR, 0.78; 95% CI, 0.70-0.88), and major adverse kidney events (aHR, 0.54; 95% CI, 0.44-0.67).
Among several limitations to the study Wu and colleagues point to the use of registry-based data which could have introduced errors that would influence the results and reliance on diagnostic codes to identify variables, which could result in misclassification and potential bias. The observational nature of the study leaves open the possibility of residual confounding, they acknowledged.
“Our findings also provide directions for future research in other target populations,” the researchers wrote. “The observational nature of our data precluded inference of causality, which might be confirmed in future research, such as in ongoing randomized controlled trials evaluating the efficacy of tirzepatide in improving CV and kidney outcomes compared with GLP-1 agonists and in treating patients with heart failure with preserved ejection fraction.”
References
1. Chuang M-H, Chen J-Y, Want H-Y, Jian Z-H, Wu V-C. Clinical outcomes of tirzepatide or GLP-1 receptor agonists in individuals with type 2 diabetes. JAMA Netw Open. 2024;7(8):e2427258. doi:10.1001/jamanetworkopen.2024.27258
2. Karagiannis T, Avgerinos I, Liakos A, et al. Management of type 2 diabetes with the dual GIP/GLP-1 receptor agonist tirzepatide: a systematic review and meta-analysis. Diabetologia. 2022;65(8):1251-1261. doi:10.1007/ s00125-022-05715-4 19
3. Wilson JM, Lin Y, Luo MJ, et al. The dual glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 receptor agonist tirzepatide improves cardiovascular risk biomarkers in patients with type 2 diabetes: a post hoc analysis. Diabetes Obes Metab. 2022;24(1):148-153. doi:10.1111/dom.14553 20
4. Jastreboff AM, Aronne LJ, Ahmad NN, et al; SURMOUNT-1 Investigators. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205-216. doi:10.1056/NEJMoa2206038
Obesity Linked to Faster Alzheimer Disease Progression in Longitudinal Blood Biomarker Analysis
December 2nd 2025Biomarker trajectories over 5 years in study participants with AD show steeper rises in pTau217, NfL, and amyloid burden among those with obesity, highlighting risk factor relevance.
