- Clinical Technology
- Adult Immunization
- Hepatology
- Pediatric Immunization
- Screening
- Psychiatry
- Allergy
- Women's Health
- Cardiology
- Pediatrics
- Dermatology
- Endocrinology
- Pain Management
- Gastroenterology
- Infectious Disease
- Obesity Medicine
- Rheumatology
- Nephrology
- Neurology
- Pulmonology
Splenic Pneumocystosis: An Atypical Presentation of Extrapulmonary Pneumocystis Infection
A 42-year-old white woman with AIDS presented to the emergency department (ED) with a 5-day history of persistent, high-grade fever (temperature of 38.3°C to 40.0°C [101°F to 104°F]); generalized weakness; malaise; and mild headache. The previous night she noted the onset of nausea, emesis, and loss of appetite.
A 42-year-old white woman with AIDS presented to the emergency department (ED) with a 5-day history of persistent, high-grade fever (temperature of 38.3°C to 40.0°C [101°F to 104°F]); generalized weakness; malaise; and mild headache. The previous night she noted the onset of nausea, emesis, and loss of appetite.
The patient's HIV infection was diagnosed in 1992, and her medical history was also significant for asthma of 10-year duration, vaginal hysterectomy for cervical dysplasia in 2001, and bacterial pneumonia in 2004. She had a long-standing history of poor adherence to antiretroviral therapy and infrequent attendance at her follow-up appointments. Her most recent HIV RNA level, obtained 7 months earlier, was 3.5 million copies/mL, and her CD4+ cell count was 24/µL. Results of a purified protein derivative tuberculin skin test and tests for antibodies to hepatitis C virus were negative.
Approximately 2 weeks before presentation, the patient restarted a previous antiretroviral regimen that included lamivudine, tenofovir, abacavir, didanosine, and ritonavir-boosted fosamprenavir. She reported that she had been adherent to this regimen but had stopped taking her medications when the fever developed. She had also restarted her dapsone prophylaxis but had discontinued that as well. The only medication that the patient continued to take was montelukast for her asthma.
At presentation in the ED, the patient was febrile but appeared healthy. She denied cough, dyspnea, chest pain, dysuria, hematuria, melena, rectal bleeding, visual changes, photophobia, and neck stiffness. Findings from the physical examination were unremarkable. Her oxygen saturation was 97% on room air. A chest radiograph showed clear bilateral fields with no evidence of pleural effusion and a normal cardiomediastinal silhouette. Results of urinalysis were unremarkable. Laboratory test results were notable for microcytic anemia with pronounced teardrop-shaped cells on smears, decreased hemoglobin level (10.3 g/dL [normal, 12.1 to 15.1]), decreased hematocrit value (30.4% [normal, 36.1 to 44.3]), and elevated lactate dehydrogenase (LDH) level (658 U/L [normal, 105 to 333]). She was hospitalized for evaluation and treatment.
On admission, the patient's differential diagnosis included immune reconstitution syndrome, Pneumocystis jiroveci pneumonia (PCP), and Mycobacterium avium complex (MAC) infection. Immune reconstitution syndrome seemed highly unlikely when the patient's CD4+ cell count at admission was found to be 8/µL and her HIV RNA level was 453,940 copies/mL. Her high LDH level supported a diagnosis of PCP, but without any pulmonary findings, this, too, seemed unlikely; however, MAC infection remained a possibility.
During the course of her hospitalization, the patient's condition gradually began to deteriorate. Although constantly febrile, she increasingly began experiencing spiking fevers and drenching night sweats. Blood cultures were negative; there was no clear source of infection. The combination of an increase in her LDH level to 984 U/L, a decline in hemoglobin level to 8.5 g/dL, and haptoglobin levels below at 6 mg/dL (normal, 27 to 139) suggested ongoing hemolysis, although results of a direct Coombs test were negative.
On the patient's third hospital day, a dry, nonproductive cough developed and she became progressively short of breath. Her oxygen saturation dropped to 90% after a brief walk around the floor, and she experienced an episode of hypoxemia. A chest radiograph and CT scan revealed no evidence of pulmonary embolus, deep venous thrombosis, or PCP, but she was given intravenous pentamidine and prednisone to cover for potential pulmonary PCP nonetheless. An echocardiogram showed a normal ejection fraction and mild mitral regurgitation but no vegetations, left atrial dilation, or left ventricular hypertrophy.
An abdominal CT scan showed an abnormally enlarged, heterogeneous spleen with innumerable, low-attenuation, hypodense lesions (Figure 1). Prominent porta hepatis lymph nodes were visualized; other lymph nodes were unremarkable. Evidence of hepatomegaly and gallbladder wall thickening was demonstrated. Discrete hepatic lesions were not seen.
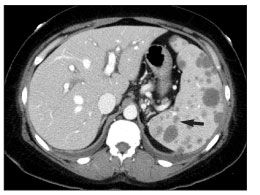
Figure 1.CT scan of the abdomen showing enlargement of the spleen (16 cm in the anterior/posterior direction) with low-attenuation lesions (arrow) and hepatomegaly (20 cm in the craniocaudal direction) with no discrete lesions. (Image courtesy of the department of nuclear medicine, New York Presbyterian Hospital–Weill Cornell Medical College.)
A bone marrow aspiration and biopsy was performed on the sixth hospital day. The aspiration sample revealed hypercellularity with increased numbers of megakaryocytes of all degrees of maturation and overall decreased erythroid activity. Myeloid maturation was normal. There were no lymphoid or plasma cell clusters. The biopsy specimen revealed additional nonspecific findings of hypercellularity and slight hypermetabolic activity, but there was no evidence of lymphoma.
Positron emission tomography/CT imaging was performed on day 8 of the patient's hospitalization, and the scan revealed abnormal, hypermetabolic activity within the enlarged, heterogeneous spleen (Figure 2). The rest of the scan was essentially unremarkable. The splenic lesions were considered to be consistent with abscesses, fungal infection, or lymphoma.
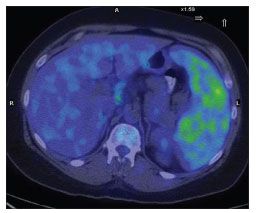
Figure 2.Positron emission tomography/CT scan showing hypermetabolic activity within lesions of an enlarged, heterogeneous spleen. (Image courtesy of the department of nuclear medicine, New York Presbyterian Hospital–Weill Cornell Medical College.)
Two weeks after admission for her constitutional symptoms, the patient underwent a diagnostic splenectomy. A diagnosis of lymphoma was ruled out by the results of pathological examination of the spleen. Repeated blood, urine, respiratory, and stool cultures remained negative, including fungal and mycobacterial cultures. Results of a serum Cryptococcus antigen test were negative. On request from the patient's attending physician, Weigert-Gram and Gomori methenamine-silver stains of the splenic specimen were found to be positive for P jiroveci (Figure 3).
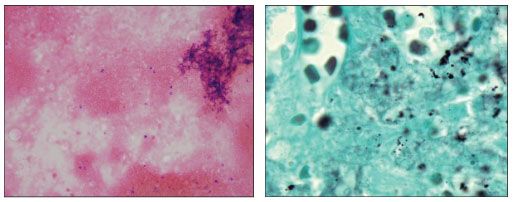
Figure 3.Demonstration of round and oval cyst walls of Pneumocystis jiroveci in the spleen (left: Weigert-Gram special stain, original magnification x400; right: Gomori methenamine-silver stain, original magnification x1000). (Images courtesy of the clinical microbiology laboratories and Dr April Chiu, department of pathology and laboratory medicine, New York Presbyterian Hospital, respectively.)
The patient's fever subsided 3 days after the splenectomy, and she was given cefazolin for postsurgical wound cellulitis. She was discharged shortly thereafter on a regimen of atovaquone and cephalexin, with guidelines for follow-up. All cultures for Mycobacterium remained negative. At a recent 3-month follow-up visit, the patient was feeling entirely well and was dedicated to taking her medication. Her most recent HIV RNA level was 408 copies/mL.
INCIDENCE AND EPIDEMIOLOGYP jiroveci (formerly known as P carinii) is a fungal, opportunistic pathogen whose clinically relevant manifestations occur almost exclusively within immunocompromised persons. Before the onset of the HIV/AIDS pandemic, fewer than 100 cases of PCP were reported annually in the United States; most of these were pulmonary events arising sporadically in high-dose corticosteroid users or in persons with acute lymphocytic leukemia.1 Cases of disseminated or extrapulmonary Pneumocystis infection are exceedingly rare. Between 1954 and 1996, our literature search could find only 16 documented cases of extrapulmonary Pneumocystis infection in the HIV-seronegative population.2
As a consequence of the HIV/AIDS pandemic, by 1990, the number of reported PCP cases had climbed to roughly 20,000 per year.3 PCP is the most common AIDS-defining opportunistic infection in the United States. Even in the HAART era, it remains a significant cause of pulmonary-related morbidity and mortality. Similarly, although still quite rare with respect to total numbers, disseminated Pneumocystis infections also began to increase in incidence, with 90 reported cases between 1982 and 1996.2
The estimated incidence rate of clinically significant disseminated Pneumocystis infection in HIV-1–infected persons ranges from 0.06% to 13%.2,4 Extrapulmonary Pneumocystis infection has been reported in patients without active pulmonary infection,5 but this is uncommon. One autopsy study from 1998 observed an incidence of extrapulmonary involvement in 13% of the patients who had PCP.4
Low clinical suspicion can lead to a failure to examine the more typical sites for dissemination. In patients who receive either systemic prophylaxis or aerosolized pentamidine treatment, disseminated manifestations may be unnoticed or unattributed to Pneumocystis if there are no overt or concurrent pulmonary signs.
In 2 studies published in 1993, postmortem examination of 2 patients, who each received systemic prophylaxis, revealed an extrapulmonary Pneumocystis infection, which suggests that extrapulmonary manifestations may break through in patients who are believed to be protected.6,7 Because of the variable clinical presentation of extrapulmonary Pneumocystis infection, the reported overall incidence of this infection likely underestimates the true scope of the disease. However, extrapulmonary Pneumocystis infection is generally accepted as a rare phenomenon despite uncertainties in calculated and reported incidences.
CLINICAL PRESENTATION
Pneumocystosis typically presents in HIV/AIDS patients who maintain a CD4+ cell count of 200/µL or less in the absence of adequate prophylaxis. Indolent, progressive dyspnea on exertion punctuated by nonproductive cough and high-grade fever (84% of patients experience temperatures greater than 38.1ºC [100.6°F])8 is the classic finding. Headache, fatigue, chills, night sweats, chest tightness or pain, and weight loss are commonly observed constitutional symptoms of PCP. Symptoms can be relatively mild, presenting subacutely, or more robust, presenting as pneumonia or respiratory failure. Approximately 7% of patients experience asymptomatic infections.9,10
Although findings on chest radiographs appear normal in 10% of cases, bilateral interstitial infiltrates progressing to an alveolar pattern are routinely demonstrated11; diffuse "ground-glass" attenuations are visualized on high-resolution CT.12 Auscultation for adventitial sounds may reveal crackles and rhonchi; however, these are nonspecific and inconclusive findings in roughly half of all cases.13 Hypoxemia, reduced PaO2, and a widened alveolar-arterial oxygen gradient are also normally evident on arterial blood gas measurements during fulminant disease.14 Extremely elevated LDH levels (greater than 450 U/L) are also suggestive of PCP if they occur within the setting of other classic PCP symptoms.15
In contrast to the typical pulmonary presentation of PCP, extrapulmonary pneumocystosis has the potential to arise in multiple tissues and can present with a broad, protean clinical picture in persons with HIV/AIDS. Postmortem analysis from various cases demonstrates that there are 3 modes of dissemination: hematogenous, lymphatic, and direct. Direct spread, for example, as a lobar infiltrate or pleural effusion,16 generally appears restricted to areas contiguous with an infected lung. Emboli containing Pneumocystis have been detected during autopsy studies of persons with disseminated infection.17 While these findings offer evidence supporting a critical role for hematogenous spread in influencing sites of extrapulmonary Pneumocystis infection, lymphatic spread has been shown to be its most significant route of dissemination.2 Analysis of the reported cases illustrates that lymphadenopathy and lymphadenitis are not uncommon manifestations of extrapulmonary Pneumocystis infection. Hilar and mediastinal lymph nodes have been shown to be leading sites for disseminated pneumocystosis.18
Persons with extrapulmonary disease generally experience a combination of overlooked symptoms related to the seeding of multiple, noncontiguous sites.2 The combination of hepatosplenomegaly and dermatological lesions caused by Pneumocystis infiltration is most commonly observed in association with pulmonary signs or lymphadenopathy; cases in which either the liver or the skin is involved have also been reported.2
A review of reported extrapulmonary Pneumocystis infections conducted by Ng and colleagues2 in 1997 detailed several exceptional sites where pneumocystosis manifested as a probable restricted infection. These sites include the thyroid, muscle, bone marrow, large bowel, meninges, cortex, and spleen. Symptoms reported by the patients in this review were remarkable and uncharacteristic for pneumocystosis even when extrapulmonary involvement was suspected. Hearing loss and ear pain were described as the chief complaints of 8 patients experiencing Pneumocystis dissemination limited to the middle or outer ear. Symptoms associated with ocular infiltration of both the retina and choroid were variable, ranging from no reported symptoms to visual field abnormalities. Given that extrapulmonary Pneumocystis infection has been seen in HIV-infected patients who had no prior episodes of PCP as well as in those who receive PCP prophylaxis6 and that there are diverse routes of Pneumocystis dissemination, a differential for symptomatic HIV-infected patients not responding to conventional therapeutic approaches should include extrapulmonary Pneumocystis infection irrespective of whether or not there exists a documented history of Pneumocystis infection.
DIAGNOSIS
Extrapulmonary pneumocystosis is rare and has a broad, variable, and nonspecific clinical presentation. For these reasons, it tends to be overlooked in favor of more widespread and common opportunistic infections, such as those caused by MAC, Mycobacteriumtuberculosis, and Cryptococcus species, as well as malignancies, such as lymphoma. Suspicion on the part of the health care provider when faced with variable symptoms facilitates the diagnosis of extrapulmonary Pneumocystis infection in the absence of active pulmonary infection, in the absence of a previous diagnosis of PCP, or in the presence of prophylaxis as a breakthrough infection. Once extrapulmonary Pneumocystis infection is suspected, multiple diagnositic techniques can be used to identify the pathogen at the site of dissemination, which makes its definitive diagnosis relatively stratightforward.
P jiroveci cannot be grown using current culture methods. Its detection is predicated on a successful sample collection for staining and the skill of the diagnostician. The standard method for diagnosing PCP is demonstration of Pneumocystis in either bronchoalveolar lavage fluid or induced sputum; testing with either specimen has a sensitivity up to 80% to 95%.19,20 Specimen collection for the diagnosis of extrapulmonary Pneumocystis infection in persons with HIV/AIDS is facilitated by affected tissues sustaining large burdens of P jiroveci with minimal inflammation.2 Thus, a histological examination that reveals an eosinophilic, foamy substance surrounding cyst walls and/or trophozoites in affected tissues is generally regarded as the primary diagnostic method for extrapulmonary pneumocytosis.21
A variety of additional histopathological stains are helpful in confirming the diagnosis. Grocott methenamine-silver (GMS), Weigert-Gram, and toluidine blue stains stain the cyst wall; Giemsa and Wright stains do not stain the cyst wall but detect both cystic and trophic forms.22 Immunofluorescent staining with a monoclonal antibody can also be employed.23 While comparable sensitivities are reported with the above-mentioned stains, specimens stained with Papanicolaou or hematoxylin-eosin are faint and fail to illustrate the characteristic morphology of cystic and trophic forms.18P jiroveci can be identified using polymerase chain reaction (PCR) assays, but a high pathogen burden in affected tissues typically makes this method unnecessary for diagnosis of disseminated disease.
TREATMENT
It has been hypothesized that PCP and extrapulmonary Pneumocystis infection are caused by genetic variants of P jiroveci that may potentially differ in their susceptibility to therapeutic regimens.24 Nonetheless, the standard of treatment for extrapulmonary Pneumocystis infection remains comparable to that for PCP. Pneumocystis therapy in HIV-infected patients typically consists of a 21-day course of an orally or intravenously (depending on disease severity) administered antimicrobial.25 Adjunctive corticosteroid treatment is also given in severe, fulminant cases (eg, hypoxemic patients with PaO2 values less than 70 mm Hg).26,27
Intravenous or oral trimethoprim/sulfamethoxazole (TMP/SMX) is the initial treatment option for pneumocystosis in HIV-infected patients who do not exhibit an allergy, intolerance, or drug failure.28 Patients receiving TMP/SMX should be closely monitored because some adverse reactions, such as hyperkalemia, hepatotoxicity, neutropenia, and GI irritation, are dose-dependent and are not uncommon. Documented mutations in the dihydropteroate synthase gene conferring sulfonamide resistance to P jiroveci is a concern with the use of TMP/SMX as a treatment and prophylactic agent.29,30 However, these mutations have not been conclusively proven responsible for regimen failure.
Pentamidine is the most effective alternative antimicrobial therapy for HIV-infected patients who are intolerant to TMP/SMX or those who have breakthrough infections.31 Pentamidine is administered intravenously in an in-patient setting with close monitoring; use of this drug has the potential for serious adverse effects, which include arrhythmias, hyperkalemia and hypokalemia, nephrotoxicity, pancreatitis, dysglycemia, and frank diabetes.32 Aerosolized pentamidine has limited effectiveness in treating PCP because of high relapse rates, and therefore it is not used.33 Aerosolized pentamidine has also been proposed to increase the risk of extrapulmo-nary Pneumocystis infection developing, but studies have shown this to be incorrect. Breakthrough Pneumocystis infection in patients who are receiving prophylaxis with dapsone, atovaquone, or aerosolized pentamidine is not uncommon.
Atovaquone suspension has been used to effectively treat patients who have mild cases of pulmonary Pneumocystis infection.34 However, as shown by Ng and colleagues,2 patients in most of the reported cases of extrapulmonary Pneumocystis infection were successfully treated with either TMP/SMX or intravenous pentamidine. Clindamycin/primaquine and TMP/dapsone were also successfully used. Previous reports have evaluated the use of trimetrexate as an adjunctive therapy to TMP/ SMX or with leucovorin for treatment of extrapulmonary Pneumocystis infection, but because of common adverse effects and treatment failures, trimetrexate is no longer available in the United States. While there exists a therapeutic hierarchy for treating pulmonary Pneumocystis infection, studies have not identified a single, specific intervention or therapy clearly associated with either a better or a worse outcome for extrapulmonary Pneumocystis infection despite this disease's variable and tissue-specific manifestations.
CONCLUSION
This case of splenic pneumocystosis was extraordinary because of its variable presentation in this patient throughout the clinical course. Given the patient's high viral load and low CD4 count, the initial clinical picture of fever, headache, malaise, nausea, and vomiting in the setting of restarted antiretroviral therapy could have resulted from a host of sources, including immune reconstitution syndrome, adverse reactions related to the resumed medications, malignancy, or a number of possible opportunistic infections. The combination of her gradual deterioration and acquisition of more severe respiratory symptoms were suggestive of PCP; yet, multiple negative tests results made that diagnosis less likely. Empirical treatment with pentamidine, lack of demonstrable pulmonary involvement, and negative lymph nodes on a CT scan further complicated consideration of extrapulmonary Pneumocystis infection. Support for the diagnosis was found only in a few nonspecific laboratory values and by direct visualization of the removed spleen.
It seems clear that the patient's Pneumocystis infection was concentrated in her spleen and that splenectomy was essentially curative. The atovaquone suspension was deemed sufficient to treat any residual, undetected infection. This case indicates that despite advances in the prophylaxis, diagnosis, and treatment of the characteristic pulmonary manifestations of pneumocystosis, infection with P jiroveci can be of considerable significance in caring for persons with HIV/AIDS because of this pathogen's rare, yet serious, potential for extrapulmonary dissemination.
References:
References1. Walzer PD, Perl DP, Krogstad DJ, et al. Pneumocystiscarinii pneumonia in the United States. Epidemiologic, diagnostic, and clinical features. Ann Intern Med. 1974;80:83-93.
2. Ng VL, Yajko DM, Hadley WK. Extrapulmonary pneumocystosis. Clin Microbiol Rev. 1997;10:401-418.
3. Centers for Disease Control and Prevention. Guidelines for prophylaxis against Pneumocystiscarinii pneumonia for persons infected with human immunodeficiency virus. MMWR. 1989;38(suppl 5):1-9.
4. Afessa B, Green W, Chiao J, Frederick W. Pulmonary complications of HIV infection: autopsy findings. Chest. 1998;113:1225-1229.
5. Kallen AJ, Wallace MR. Atypical Pneumocystiscarinii infection in AIDS: massive cervical lymphadenitis and fever of unknown origin. South Med J. 1998;91:759-760.
6. Carlin EM, Coker RJ, Goldin RD, Harris JR. Systemic Pneumocystiscarinii pneumonia prophylaxis with dapsone and pyrimethamine fails to protect against extrapulmonary pneumocystosis. Genitourin Med. 1993;69:130-132.
7. Podzamczer D, Martos A, Ferrer I, et al. Hepatic and pulmonary pneumocysÂtosis during primary prophylaxis for Pneumocystiscarinii pneumonia with dapsone/pyrimethamine. Clin Infect Dis. 1993;16:171.
8. Kales CP, Murren JR, Torres RA, Crocco JA. Early predictors of in-hospital mortality for Pneumocystiscarinii pneumonia in the acquired immunodeficiency syndrome. Arch Intern Med. 1987;147:1413-1417.
9. Barron TF, Birnbaum NS, Shane LB, et al. Pneumocystiscarinii pneumonia studied by gallium-67 scanning. Radiology. 1985;154:791-793.
10. Engelberg LA, Lerner CW, Tapper ML. Clinical features of Pneumocystis pneumonia in the acquired immune deficiency syndrome. Am Rev Respir Dis. 1984;130:689-694.
11. Opravil M, Marincek B, Fuchs WA, et al. Shortcomings of chest radiography in detecting Pneumocystiscarinii pneumonia. J Acquir Immune Defic Syndr. 1994;7:39-45.
12. Gruden JF, Huang L, Turner J, et al. High-resolution CT in the evaluation of clinically suspected Pneumocystiscarinii pneumonia in AIDS patients with normal, equivocal, or nonspecific radiographic findings. AJR. 1997;169:967-975.
13. Selwyn PA, Pumerantz AS, Durante A, et al. Clinical predictors of PneumoÂcystis carinii pneumonia, bacterial pneumonia and tuberculosis in HIV-infected patients. AIDS. 1998;12:885-893.
14. Smith DE, McLuckie A, Wyatt J, Gazzard B. Severe exercise hypoxaemia with normal or near normal X-rays: a feature of Pneumocystiscarinii infection. Lancet. 1988;2:1049-1051.
15. Zaman MK, White DA. Serum lactate dehydrogenase levels and Pneumocystiscarinii pneumonia. Diagnostic and prognostic significance. Am Rev Respir Dis. 1988;137:796-800.
16. Dyner TS, Lang W, Busch DF, Gordon PR. Intravascular and pleural involvement by Pneumocystiscarinii in a patient with the acquired immunodeficiency syndrome (AIDS). Ann Intern Med. 1989;111:94-95.
17. Davey RT Jr, Margolis D, Kleiner D, et al. Digital necrosis and disseminated Pneumocystiscarinii infection after aerosolized pentamidine prophylaxis. Ann Intern Med. 1989;111:681-682.
18. Anuradha, Sinha A. Extrapulmonary Pneumocystiscarinii infection in an AIDS patient: a case report. Acta Cytol. 2007;51:599-601.
19. Baughman RP. Current methods of diagnosis. In: Walzer PD, ed. Pneumocystis carinii Pneumonia. 2d ed. New York: Dekker; 1994:381-401.
20. Huang L, Hecht FM, Stansell JD, et al. Suspected Pneumocystiscarinii pneumonia with a negative induced sputum examination. Is early bronchoscopy useful? Am J Respir Crit Care Med. 1995;151:1866-1871.
21. Cregan P, Yamamoto A, Lum A, et al. Comparison of four methods for rapid detection of Pneumocystiscarinii in respiratory specimens. J Clin Microbiol. 1990;28:2432-2436.
22. Centers for Disease Control and Prevention. Treating opportunistic infections among HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association/Infectious Diseases Society of America. MMWR. 2004;53(RR-15):5-8.
23. Ng VL, Virani NA, Chaisson RE, et al. Rapid detection of Pneumocystiscarinii using a direct fluorescent monoclonal antibody stain. J Clin Microbiol. 1990;28:2228-2233.
24. Lecuit M, Livartowski J, Vons C, et al. Resistance to trimethoprim-sulfamethoxazole and sensitivity to pentamidine therapy in an AIDS patient with hepatosplenic pneumocytosis. AIDS. 1994;8:1506-1507.
25. Wharton JM, Coleman DL, Wofsy CB, et al. Trimethoprim-sulfamethoxazole or pentamidine for Pneumocystiscarinii pneumonia in the acquired immunoÂdeÂfiÂciency syndrome. A prospective randomized trial. Ann Intern Med. 1986;105:37-44.
26. Bozzette SA, Sattler FR, Chiu J, et al. A controlled trial of early adjunctive treatment with corticosteroids for Pneumocystiscarinii pneumonia in the acquired immunodeficiency syndrome. California Collaborative Treatment Group. N Engl J Med. 1990;323:1451-1457.
27. Gallant JE, Chaisson RE, Moore RD. The effect of adjunctive corticosteroids for the treatment of Pneumocystiscarinii pneumonia on mortality and subsequent complications. Chest. 1998;114:1258-1263.
28. Safrin S, Finkelstein DM, Feinberg J, et al. Comparison of three regimens for treatment of mild to moderate Pneumocystiscarinii pneumonia in patients with AIDS. A double-blind, randomized, trial of oral trimethoprim-sulfamethoxazole, dapsone-trimethoprim, and clindamycin-primaquine. ACTG 108 Study Group. Ann Intern Med. 1996;124:792-802.
29. Helweg-Larsen J, Benfield TL, Eugen-Olsen J, et al. Effects of mutations in Pneumocystiscarinii dihydropteroate synthase gene on outcome of AIDS-associÂated P. carinii pneumonia. Lancet. 1999;354:1347-1351.
30. Huang L, Crothers K, Atzori C, et al. Dihydropteroate synthase gene mutations in Pneumocystis and sulfa resistance. Emerg Infect Dis. 2004;10:1721-1728.
31. Sattler FR, Cowan R, Nielsen DM, Rushkin J. Trimethoprim-sulfamethoxazole compared with pentamidine for treatment of Pneumocystiscarinii pneumonia in the acquired immunodeficiency syndrome. A prospective, noncrossover study. Ann Intern Med. 1988;109:280-287.
32. Liegl U, Bogner JR, Goebel FD. Insulin-dependent diabetes mellitus following pentamidine therapy in a patient with AIDS. Clin Investig. 1994;72:1027-1029.
33. Montgomery AB, Feigal DW Jr, Sattler F, et al. Pentamidine aerosol versus trimethoprim-sulfamethoxazole for Pneumocystiscarinii in acquired immune deficiency syndrome. Am J Respir Crit Care Med. 1995;151:1068-1074.
34. Kovacs JA, Gill VJ, Meshnick S, Masur H. New insights into transmission, diagnosis, and drug treatment of Pneumocystiscarinii pneumonia. JAMA. 2001;286:2450-2460.
Â
