- Clinical Technology
- Adult Immunization
- Hepatology
- Pediatric Immunization
- Screening
- Psychiatry
- Allergy
- Women's Health
- Cardiology
- Pediatrics
- Dermatology
- Endocrinology
- Pain Management
- Gastroenterology
- Infectious Disease
- Obesity Medicine
- Rheumatology
- Nephrology
- Neurology
- Pulmonology
The Significance of Progression-Free Survival as a Clinical End Point in Oncology
In the metastatic setting, given the limited potential for a cure, treatment is focused on extending patient life, providing symptom relief, and improving quality of life.
It is challenging to develop effective and safe treatments for cancer in a cost-effective and timely manner. To gain FDA approval of new agents, it is necessary for cancer trials to enroll large numbers of patients, allowing observation of sufficiently significant differences in clinical benefit at various survival end points. Here we discuss the relative merits of 2 survival end points-overall survival (OS) and progression-free survival (PFS)-and the value of PFS from the managed care perspective.
The detection of differences in survival end points between the comparator treatment and the investigational agent can require a long period for evaluation. This is especially seen in patients with certain tumor types who are expected to benefit from a long period of survival. Conducting trials in these patients with OS as the primary end point can ultimately result in greater associated costs. This is particularly true for cancers for which the survival measure has improved.
A report by Sargent and Hayes1 highlighted this issue with the example of metastatic breast cancer. Over the past 20 years, OS has increased substantially for patients with metastatic breast cancer. The OS improved from approximately 14.5 months for patients who received a diagnosis in 1991 to approximately 22 months for women who received a diagnosis in 2001.
Currently, median metastatic breast cancer OS is approximately 26 months. As median survival continues to increase, the sample size necessary to conclusively identify the same absolute improvement in survival (eg, 3 months) increases considerably. In addition, observation and reporting of OS may take several years beyond the detection of other survival end points, such as PFS.2 Going forward, such considerations will become more prominent in the conduct of clinical trials.
Booth and colleagues3 conducted an analysis of 326 randomized controlled trials (RCTs) published in 6 journals between 1975 and 2004 for several cancers with high rates of incidence, such as breast, colorectal, and non–small-cell lung cancers (NSCLCs). Results showed that survival was increasingly used as the primary trial end point, from 13% to 48%. Use of response rate decreased from 32% to 14%. The number of patients enrolled in RCTs also increased over this period-an average of 100 patients were enrolled in RCTs from 1974 to 1984, and this increased to an average of 441 patients from 1995 to 2004.
Thus, RCTs in oncology have become more common and larger, with increasing use of OS as the primary end point. Such trends in clinical trial design have consequences for all participants in the health care system: the pharmaceutical and biotechnology companies that develop the drugs, health plans that reimburse treatment costs, and patients and oncologists who are impacted by costs.
OS as Clinical Measure
Currently, OS is the most commonly used clinical end point in the metastatic setting for phase 3 RCTs and regulatory approval.4 An analysis of 230 clinical trials from the American Society of Clinical Oncology meeting reports from 2003 to 2007 revealed that OS was the primary end point in 51% of all trials of cytotoxic agents and in 54% of all trials of molecular-targeted agents. In studies of advanced-stage disease, PFS was the primary end point in 23% of RCTs of molecular-targeted agents and in 15% of RCTs of cytotoxic agents.5
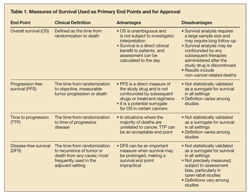
OS is defined as the time from randomization in the clinical trial to death, so it is unambiguous and not open to interpretation by the investigator (Table 1). The patient benefit can be described as superior survival or noninferior survival after consideration of toxicity and the magnitude of benefit. A noninferiority analysis ensures that a survival advantage associated with an approved drug will not be lost with a new agent.2,4 However, OS as a primary end point for clinical trials has several major drawbacks.
Study duration and number of patients. Because death may occur after a relatively long time for some malignancies, especially those with an indolent clinical course, the reliable detection of statistically significant differences in OS
requires large numbers of patients and several years.1
In a recent trial, women with metastatic breast cancer were randomly assigned to receive either bevacizumab or no bevacizumab in addition to first-line paclitaxel. The median OS for the trial population was not reached until nearly 8 years after the trial was opened. This trial end point was reached 2 years after initial results based on an end point of PFS were reported.
Similarly, OS results for the phase 3 trial of sunitinib or interferon (IFN) as first-line treatment of renal cell carcinoma (RCC) were also obtained approximately 2 years after the first determination of the PFS end point.6,7 This is particularly important in cancers and hematological malignancies such as prostate cancer, certain subtypes of non-Hodgkin lymphoma, and chronic lymphocytic leukemia that have a long indolent course and for which measurement of survival may take many years.
Crossover of patients. Another significant disadvantage for OS as an end point concerns the crossover of patients from the initial control, or comparator, arm. This is increasingly important with many new investigational agents. Additional crossover is observed with disease progression and off-study administration of the investigational drug.
This is an appropriate management strategy, but unfortunately it reduces the ability to measure OS related to a specific treatment because patients in both study arms receive the new agent at different points. Although a prohibition of such crossover may appear “scientifically” appealing, ethical considerations preclude this approach, particularly if outcomes on earlier end points are found to be promising or the drug is approved in later-line settings.
Recent clinical data have shown a survival benefit for patients with NSCLC harboring epidermal growth factor receptor (EGFR) mutations who were treated with anti-EGFR therapy. Results of the phase 3 trial First-line Single Agent Iressa versus Gemcitabine and Cisplatin Trial in Never-smokers With Adenocarcinoma of the Lung (First-SIGNAL),8 which compared gefitinib with standard chemotherapy in never-smoker patients, showed a significant improvement in the 1-year PFS rate. However, no significant improvement was shown in OS, the primary end point. This is most likely because 80.7% of the patients receiving chemotherapy also received treatment with EGFR therapy after disease progression.
In contrast, an open-label study of patients with EGFR mutations treated with erlotinib showed a highly significant improvement in the median PFS of 14 months as well as an improvement in median OS of 27 months.9
One approach to mitigate such confounding effects would be to identify and censor such crossover effects in analyses of survival data. An example of this approach is in a recent report on the survival results for patients with advanced RCC who were treated with sunitinib or IFN.7 The median OS for patients treated with sunitinib or IFN was 26.4 months compared with 21.8 months for IFN alone. An exploratory analysis of the OS benefit, involving censorship of crossover patients from the IFN arm of the study, showed a further increase in OS benefit: 26.4 months versus 20 months.
Other treatment options. Finally, improvement in OS may be more difficult to detect in studies of new agents now than in the past because of the beneficial effects of the many other therapies now available for cancers such as breast cancer. Improved OS rates resulting from the current standard of care for the particular cancer are associated with larger clinical trials, which are needed to detect statistically significant differences between the treatment arms for investigational agents.
PFS as End Point
PFS is the other survival end point commonly used to determine clinical efficacy in RCTs and for drug approval.4 PFS is defined as the time from randomization of the patients in a trial to objective, measurable tumor progression or death (Table 1).
Benefits and disadvantages. Because PFS includes death in its measurement, it is considered to be a better correlate to OS than time to progression (TTP). TTP is an end point that only considers the time from randomization to the time of progressive disease.8,10 PFS considers and allows subsequent lines of treatment without confounding the measurement, thus creating a cleaner end point.
A clear disadvantage of measuring PFS is the inability to precisely determine the time point of progression of disease. This potential inconsistency could result in overestimating the benefit of treatment.11,12 Standardization of the protocol to measure PFS can alleviate this concern. This may include an increased frequency of scans in RCTs to identify evidence of disease progression in a patient.
Bias in evaluation time may be minimized if trials can be performed as placebo-controlled double-blind studies and the blinding is effective.12 But again, this is a difficult process, with clear intra-trial data analysis requirements. In nonblinded trials, more frequent evaluations of disease progression would lessen any evaluation-time bias.
Evaluation frequency should also be considered when measuring median PFS rates. For example, evaluation-time bias may occur if patients are evaluated every 3 months, yet current median PFS rates were 4 months for a specific cancer. Another excellent strategy is to compare PFS rates at a single time point; for example, 6-month PFS rates could be compared between the treatment arms.12
The value of PFS as end point. The results of a study that compared paclitaxel with paclitaxel plus bevacizumab for the treatment of breast cancer recently highlighted the issues surrounding the value of PFS as end point in the clinical evaluation of promising new therapies. In this study, PFS was significantly improved and almost doubled, from 5.9 months to 11.8 months, in patients treated with the bevacizumab-containing regimen. However, the argument was made that there was no statistically significant improvement in OS.13 The improvement in 1-year survival with the addition of bevacizumab (bevacizumab, 81.2% vs no bevacizumab, 73.4%; P = .01) confirmed the PFS benefit.13 However, the trial was only 80% powered to detect a 7-month improvement in OS.
There also has been a debate on the value of PFS as a predictor, or surrogate, for OS. The potential advantage of using PFS as a surrogate for OS is that it would allow for the evaluation of trial drugs in a more rapid manner. Retrospective analyses of RCTs in patients with metastatic colorectal cancer have reported that improvement in PFS is strongly correlated with improvement in OS.14,15
A similar correlation between 6-month PFS and 12-month OS was determined from a number of studies in patients with glioblastoma multiforme. PFS is considered a valid end point in this disease.16-18
However, this correlation may be inconsistent with other cancers. This was demonstrated by an analysis of 11 RCTs comparing anthracycline with taxane chemotherapy in patients with metastatic breast cancer. This study failed to find a correlation for surrogacy of PFS for OS.19
Such variability may indicate that the surrogacy of PFS for OS, and end points in general, should be considered on a case-by-case basis for each specific disease state, given the number of cancers and their individual pathophysiological complexity. In addition, the evaluation of PFS and OS may be affected by the same factors that could mask any correlation between the two end points.
Improvement in PFS may be considered to be maximizing disease control and improving quality of life (QoL) for patients. For as long as possible, patients are spared from the symptoms of progressive disease, undergoing further treatment with additional therapies and their attendant toxicities, and the psychological burden and uncertainty that come with disease progression. A phase 3 RCT using panitumumab to treat patients with refractory metastatic colorectal cancer demonstrated that the lack of disease progression was associated with better symptom control, QoL, and OS.20 This implies potential cost savings associated with prolonged PFS.
The Managed Care Perspective
In managed care, many of these issues concerning survival end points are barely considered, if not ignored. The value of these end points is difficult to measure, because considerations of both PFS and OS must intersect financial considerations. A simple example here is the value of aspirin versus the value of other antithrombotic therapy. Aspirin has one of the highest returns on investment for medicine, yet it is rarely addressed within benefit designs.
Managed care struggles to measure the value of a single treatment for many diseases. Considering PFS is a difficult concept. It requires the application of preventive cancer treatment in every stage and for every cancer. The judgment of value forces the question, “Can aggressive early intervention prolong if not eliminate progression in a particular disease?”
A cost comparison of progressive disease versus progression-free disease yields obvious results. When curative (versus palliative) treatment is initiated, disease progression always leads to escalation of therapy. This escalation of therapy consumes more financial resources, which is seen in the following instances:
• Patient with stage II breast cancer recurrence who had no previous radiotherapy may now require radiotherapy and second-line chemotherapy.
• Patient with previous breast-conserving surgery may require total mastectomy or wide local excision with neoadjuvant chemotherapy.
• Patient with recurrent NSCLC requires postoperative external beam radiation therapy (dosing is dependent on nodal involvement and resection margin pathology). Local recurrence requires surgical resection and evaluation for chemoradiation.
• Patient with confirmed and resectable colon cancer recurrence requires a multidisciplinary approach to evaluate surgical interventions and systemic chemotherapy strategies (and perhaps KRAS mutation analysis).
It is irrefutable that the prevention of cancer is superior to early detection. What is not well understood, or appreciated, by the health care industry is in which case aggressive therapeutic investments should be made to optimize clinical outcomes, productivity, QoL, and financial returns in patients with early-stage to various later-stage cancers.
Unfortunately, in many health plans, the horizon of return (how long to achieve the desired return on investment) skews this critical question. Relatively short horizons lead to considering and balancing aggressive high-cost treatment with variables such as the likelihood of renewal or disenrollment, government program eligibility, or even death within a fixed time frame.
Many times within the health care finance industry, these investments must have desirable outcomes within a very short time frame. It is completely inappropriate to measure these returns in such a short period. This phenomenon is not exclusive to cancer care, but it is a setting in which the stakes and emotions are extraordinarily high.
The flu vaccine fits nicely into the complex return equation, with clear returns in very short time frames. However, in cancer and other complex chronic diseases, such as multiple sclerosis and rheumatoid arthritis, the answers are elusive and often anchored in OS or acute care avoidance only. A month of progression-free disease is invariably less expensive than a month of aggressive treatment for any progressive disease.
Developing a theoretical model of PFS. A theoretical model of PFS should accommodate time and average per patient per month expenditures. This should be compared with costs related to patients’ disease progressing to more advanced stages. The model should also accommodate the overall life expectancy and age of the patient at diagnosis. This allows the calculation of medical benefit ratios (MBRs) for patients with and for those without progressive disease and the ultimate financial return.
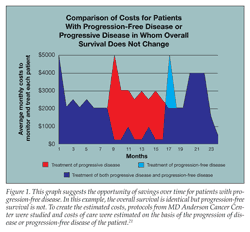
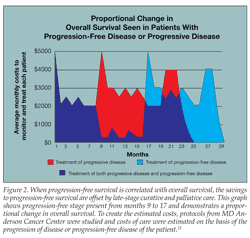
Progression-free disease clearly yields improved MBRs compared with progressive disease and expanded use of expensive and aggressive therapies. Figure 1 suggests the opportunity of savings over time for PFS. When PFS is correlated with OS, the savings to PFS are offset by late-stage curative and palliative care (Figure 2).
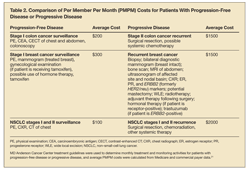
Estimated per member per month (PMPM) costs for several cancers are shown in Table 2. The scenarios use average costs for therapy or surveillance and are based on large commercial and Medicare database claims in accordance with MD Anderson Cancer Center’s published protocols for the patient descriptions. To create the estimated costs, protocols from MD Anderson Cancer Center were studied and costs of care were estimated on the basis of the progression of disease or progression-free disease of the patient.21
Economic burden of disease progression. Managed care organizations rightfully focus on the review of high-cost interventions. However, in most circumstances, the reviews and ultimate decisions for coverage are based on data derived from studies with specific end points. With cancer
care, this equates to OS only. The notion of aggressive early therapy leading to PFS and lower PMPM costs is not considered. For many chronic diseases, this is the case. However, the average cost of providing health care services for a patient with cancer in remission or a patient who is progression-free is lower than the cost of providing care for a cancer patient in need of escalating therapy.
Two studies that estimated the costs associated with lung cancer highlight the economic burden of disease progression.22,23 One retrospective case-control cohort study using a database containing inpatient, outpatient, and drug claims for patients found that the failure of initial treatment was associated with markedly increased costs.22
The monthly initial treatment phase costs of $11,496 per patient were higher than the $3733 costs during the secondary treatment phase or the $9399 costs in the terminal care phase. However, patients who experienced treatment failure accrued an additional $10,370 per month in initial treatment phase costs and $8779 more per month after starting the secondary treatment phase and/or the terminal care phase, compared with patients who required only initial treatment.
The total treatment cost for patients who experienced treatment failure was $120,650 compared with $45,953 for those who had received initial treatment only. The results showed that incremental costs associated with treatment failure were $19,149 per month and $74,697 across the study period.
A second retrospective study of 306 patients with stage III or IV metastatic NSCLC estimated the mean costs for the 3-month period after progression.23 Of the 306 patients who received chemotherapy, 108 experienced progressive disease. The total cost of care from progression to death or to the end of the study was $42,066. The mean direct 3-month postprogression cost of care was $31,129 for patients with progressive disease compared with $18,802 for patients with stable disease, yielding an incremental cost of $12,327.
Summary
The economic burden of cancer care on the US health care system is significant and growing. However, improved prevention strategies, new therapies, and adjuvant treatment may reduce both the use of health care resources and costs. Disease progression in cancer patients is costly and will affect health care budgets; costs will increase as new treatments continue to redefine cancer as a chronic disease.
Aggressive and early treatment is warranted in the metastatic setting, and current clinical data suggest that halting disease progression is associated with the clinical benefit of sustained or improved QoL and prolonged survival in patients with certain cancers. Clinical trials will be necessary to prospectively test such associations. Given the clinical complexity and statistical issues with OS and cancer and the economic benefits of progression-free disease, it is imperative to consider a measure of aggressive cancer therapy utilizing PFS and begin a new approach to successful cancer therapy.
AcknowledgmentSupport for third-party writing assistance for this article was provided by Genentech, Inc, San Francisco.
References:
References
1. Sargent DJ, Hayes DF. Assessing the measure of a new drug: is survival the only thing that matters? J Clin Oncol. 2008;26:1922-1923.
2. Pazdur R. Endpoints for assessing drug activity in clinical trials. Oncologist. 2008;13(suppl 2):19-21.
3. Booth CM, Cescon DW, Wang L, et al. Evolution of the randomized controlled trial (RCT) in oncology over three decades. J Clin Oncol. 2007;25 (18 suppl). Abstract 6515.
4. US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. Guidance for industry: clinical trial endpoints for the approval of cancer drugs and biologics. http://www.fda.gov/downloads/Drugs/GuidanceCompliance RegulatoryInformation/Guidances/ucm071590.pdf. May 2007. Accessed February 12, 2010.
5. Lee L, Chen EX. Phase III trials in the molecularly targeted era: characteristics of randomized clinical trials of cytotoxic versus molecularly targeted agents. J Clin Oncol. 2008;26(15 suppl). Abstract 6641.
6. Motzer RJ, Hutson TE, Tomczak P, et al. Phase III randomized trial of sunitinib malate (SU11248) versus interferon-alfa (IFN-α) as first-line systemic therapy for patients with metastatic renal cell carcinoma (mRCC). J Clin Oncol. 2006;24(18 suppl). Abstract LBA3.
7. Motzer RJ, Figlin RA, Hutson TE, et al. Sunitinib versus interferon-alfa (IFN-α) as first-line treatment of metastatic renal cell carcinoma (mRCC): updated results and analysis of prognostic factors. J Clin Oncol. 2008;26(suppl). Abstract 5024.
8. Jin S, Lee JS, Park K, et al. First-line single agent Iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. Presented at: 13th World Lung Conference on Lung Cancer; July 31-August 4, 2009; San Francisco.
9. Rosell R, Moran T, Queralt C, et al; Spanish Lung Cancer Group. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958-967.
10. Fleming TR, Rothmann MD, Lu HL. Issues in using progression-free survival when evaluating oncology products. J Clin Oncol. 2009;27: 2874-2880.
11. Panageas KS, Ben-Porat L, Dickler MN, et al. When you look matters: the effect of assessment schedule on progression-free survival. J Natl Cancer Inst. 2007;99:428-432.
12. Freidlin B, Korn EL, Hunsberger S, et al. Proposal for the use of progression-free survival in unblinded randomized trials. J Clin Oncol. 2007;25: 2122-2126.
13. Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666-2676.
14. Tang PA, Bentzen SM, Chen EX, Siu LL. Surrogate end points for median overall survival in metastatic colorectal cancer; literature-based analysis from 39 randomized controlled trials of first-line chemotherapy. J Clin Oncol. 2007;25: 4562-4568.
15. Buyse M, Burzykowski T, Carroll K, et al. Progression-free survival is a surrogate for survival in advanced colorectal cancer. J Clin Oncol. 2007; 25:5218-5224.
16. Lamborn KR, Yung WKA, Chang SM, et al; North American Brain Tumor Consortium. Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol. 2008;10:162-170.
17. Ballman KV, Buckner JC, Brown PD, et al. The relationship between six-month progression-free survival and 12-month overall survival end points for phase II trials in patients with glioblastoma multiforme. Neuro Oncol. 2007;9:29-38.
18. Levin VA, Ictech S, Hess KR. Impact of phase II trials with progression-free survival as end-points on survival-based phase III studies in patients with anaplastic gliomas. BMC Cancer. 2007;7:106-113.
19. Burzykowski T, Buyse M, Piccart-Gebhart MJ, et al. Evaluation of tumor response, disease control, progression-free survival, and time to progression as potential surrogate end points in metastatic breast cancer. J Clin Oncol. 2008;26: 1987-1992.
20. Siena S, Peeters M, Van Cutsem E, et al. Association of progression-free survival with patient-reported outcomes and survival: results from a randomised phase 3 trial of panitumumab. Br J Cancer. 2007;97:1469-1474.
21. MD Anderson Cancer Center. Guidelines by disease site. http://utm-ext01a.mdacc.tmc.edu/mda/cm/cwtguide.nsf/luhtml/sidebar1. Accessed February 22, 2010.
22. Kutikova L, Bowman L, Chang S, et al. The economic burden of lung cancer and the associated costs of treatment failure in the United States. Lung Cancer. 2005;50:143-154.
23. Fox KM, Brooks JM, Kim J. Metastatic non-small cell lung cancer: costs associated with disease progression. Am J Managed Care. 2008;14:565-571.
