- Clinical Technology
- Adult Immunization
- Hepatology
- Pediatric Immunization
- Screening
- Psychiatry
- Allergy
- Women's Health
- Cardiology
- Pediatrics
- Dermatology
- Endocrinology
- Pain Management
- Gastroenterology
- Infectious Disease
- Obesity Medicine
- Rheumatology
- Nephrology
- Neurology
- Pulmonology
Rheumatologists Embrace Biologics Despite Coverage Limits
As the number of biologic agents for rheumatoid arthritis (RA)-and rheumatologists’ use of these agents for RA and other rheumatological diseases-grows, getting coverage for RA treatment is taking an increasing investment of physicians’ staff time. However, the majority of respondents to a recent survey say it is an investment in patient care that is worth making.
The treatment of persons with rheumatoid arthritis (RA) and related conditions received a boost with the development and FDA approval of several biologic therapies. The recently launched therapies, which are either injected or infused, target components of the aberrant immune response that causes damage to the joints in persons with RA, often slowing or even halting joint destruction. As such, these agents provide an alternative approach for those whose disease is not controlled by anti-inflammatory agents or disease-modifying drugs. About two-thirds of persons with RA for whom biologics are prescribed respond favorably to them,1 with most experiencing remission of their disease. RA affects an estimated 1.5 million Americans.2
Rheumatologists have been eager to prescribe the new agents, particularly for patients who have not responded well to other therapies. In a survey completed by 98 rheumatologists practicing in the United States, all said they prescribe biologic agents for select patients-and not just for those with RA. Several biologics have also received FDA approval for ankylosing spondylitis and psoriatic arthritis, with similar positive results.
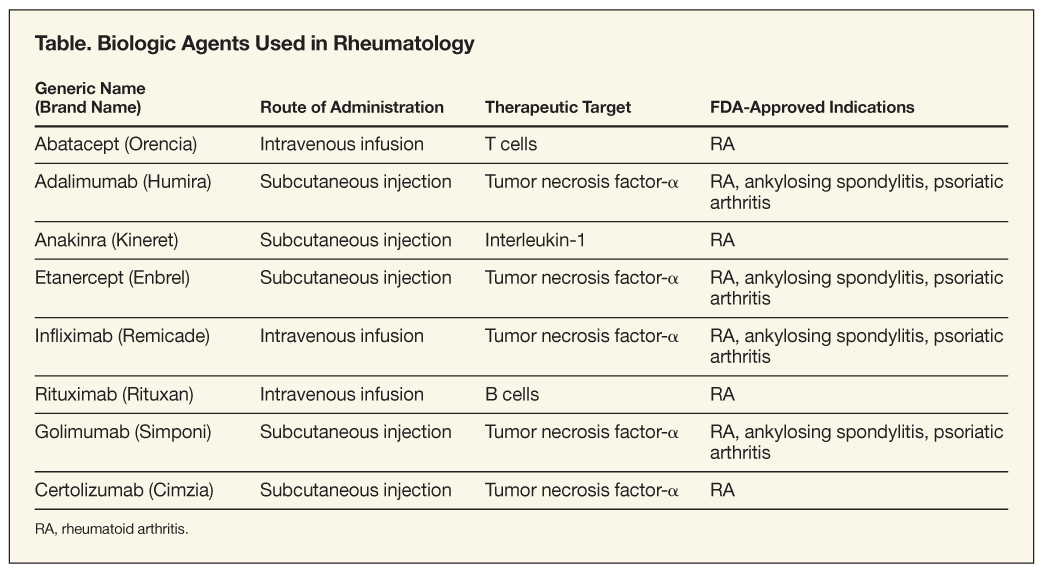
To date, the FDA has approved 8 biologic agents for RA: abatacept (Orencia), adalimumab (Humira), anakinra (Kineret), etanercept (Enbrel), infliximab (Remicade), rituximab (Rituxan) and, most recently, golimumab (Simponi) and certolizumab (Cimzia). Golimumab, etanercept, adalimumab, and infliximab are also indicated for ankylosing spondylitis and psoriatic arthritis (Table).
The trend toward prescribing these agents in rheumatology is growing, according to results of the survey Biologics Trend Report, volume 1, sponsored by UCB, Inc, the National Association of Managed Care Physicians, and the Arthritis
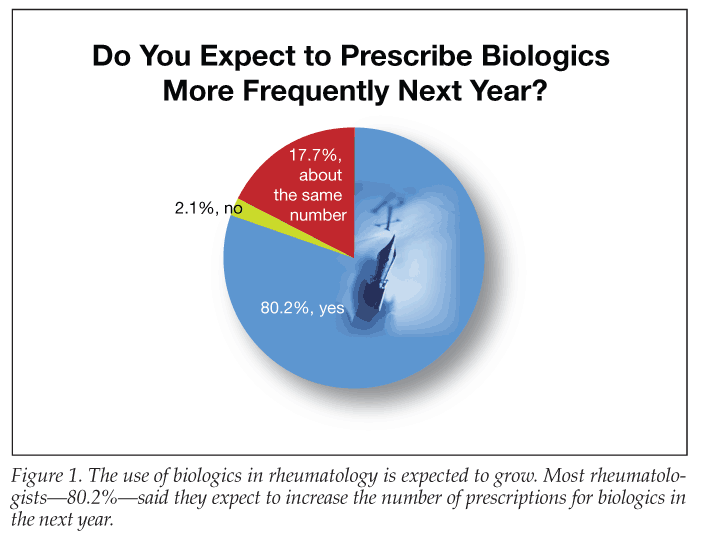
Foundation.3 Among rheumatologists responding to the survey, 76.3% said they have written more prescriptions for biologics during the past 12 months than during the preceding 12 months. About 80% said they expect to increase the number of prescriptions for biologics in the next year (Figure 1).
“Everybody is writing the same number of prescriptions for biologics or more,” said William Harrell, MD, a rheumatologist in private practice in Durham, NC, and one of the survey respondents. He expects the use of biologics to continue to rise.
Increasing Costs
However, with the rise of the new technology comes a cost-and headaches for physicians trying to get coverage of those new drugs for their patients. Biologic agents are expensive because of the high cost of raw materials and the complex manufacturing processes required for live organisms. Furthermore, the current biologics are relatively new and are still under patent protection. In addition, there is no regulatory pathway available for biogenerics or biosimilars as yet.
A year’s supply of any of the biologics can easily cost upward of $20,000-significantly more than other arthritis medications on the market. For medications that must be infused, there are additional administration costs. Without insurance coverage, few patients could afford to take them. For many, even coinsurance amounts can be a significant barrier to their use.
Physicians, however, argue that the cost of not using the new agents when indicated may be even greater, noting that RA that is allowed to progress can lead to irreparable joint damage, disability, reduced productivity and quality of life and, potentially, the need for surgery. “We know that use of the biologics can reverse and prevent a lot of damage and morbidity,” said David I. Weiss, MD, a rheumatologist practicing in Hattiesburg, Miss, and another survey respondent. Failure of health plans to cover biologics or physicians to prescribe them because of cost is shortsighted, he said. “These medications have a huge impact on preventing future problems.”
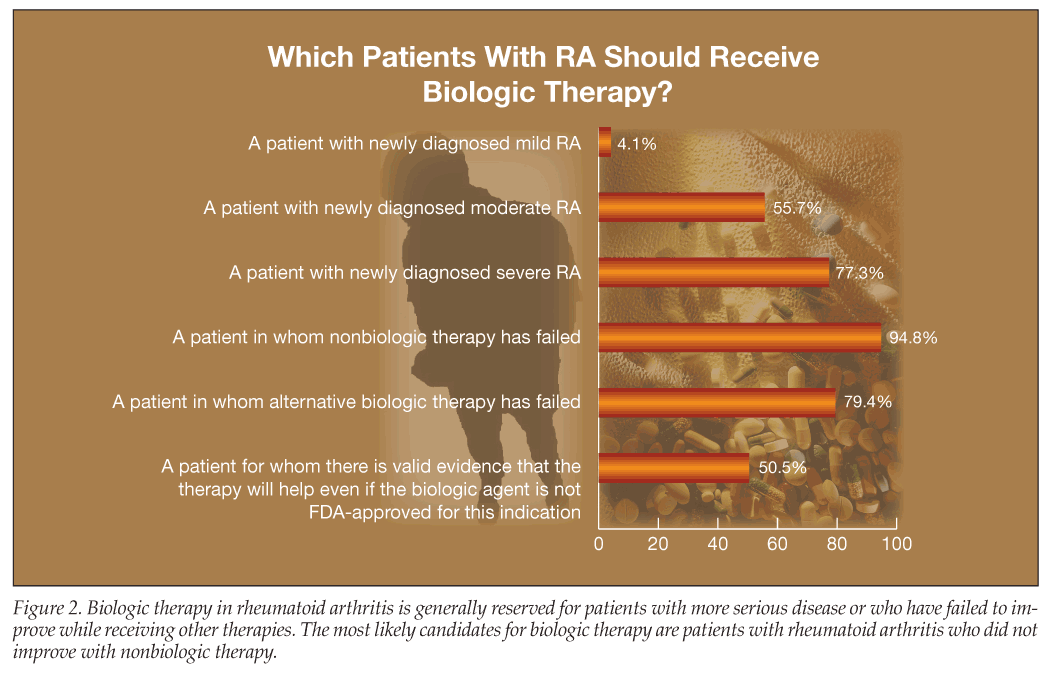
The most likely candidates for biologic therapy are patients who have been treated with nonbiologic therapy but have not improved; 94.8% of survey respondents would give such patients a biologic agent (Figure 2).
Cost is rarely a deterrent to physicians in prescribing biologics, the survey shows. In fact, recognizing the long-term consequences of inadequately treated disease and the ability of the biologics to control disease for many, rheumatologists are tending to prescribe them ever earlier in the disease process. When the first biologics came into use in rheumatology a decade ago, they were typically reserved for patients whose disease had not responded to any other treatment. Now, physicians are not waiting long to see whether other treatments are effective before prescribing a biologic agent.
For Weiss, this is especially true when it comes to psoriatic arthritis. “Patients with psoriatic arthritis can be difficult to manage because they get a lot of joint damage,” said Weiss. “So we have learned to be more aggressive with more aggressive disease.” For patients with newly diagnosed moderate psoriatic arthritis, a rheumatologist might prescribe a drug such as methotrexate on their first visit and even consider a biologic the next time they come in, Weiss added.
“Rheumatologists tend to prescribe biologics earlier for persons with psoriatic arthritis or ankylosing spondylitis because there are fewer effective nonbiologic agents for them,” said Diana Titova, MD, a rheumatologist practicing in Lakewood, Wash. “While methotrexate, a disease-modifying drug, is often effective for RA, it does not work as well for spondylarthropathies such as ankylosing spondylitis, and persons with psoriatic arthritis may be more susceptible to liver toxicity from methotrexate.”
Among rheumatologists responding to the survey, 77.1% would prescribe a biologic for a patient with newly diagnosed severe psoriatic arthritis and 61.5% would prescribe a biologic for newly diagnosed moderate psoriatic arthritis. The percentages for RA were similar: 77.3% for newly diagnosed severe RA and 55.7% for newly diagnosed moderate RA. For ankylosing spondylitis, those percentages were even higher: 85.4% for newly diagnosed severe ankylosing spondylitis and 74% for newly diagnosed moderate ankylosing spondylitis.
“For 74% of patients with newly diagnosed moderate ankylosing spondylitis to be treated with a biologic seems high,” said survey respondent Jack Waxman, MD, a rheumatologist in Santa Rosa, Calif. “It makes sense to consider a biologic for a patient who was treated with nonbiologic therapies for 6 or 12 months with no improvement seen. But I would not give a biologic right off the bat. Mild ankylosing spondylitis would be treated with NSAIDs.”
Three of the approved biologics-abatacept, infliximab, and rituximab-are given by infusion. Most rheumatology practices responding to the survey (81%) have facilities to handle infusions. Other responses included partnering with a hospital outpatient infusion center (13%), referring patients to an infusion center (8%), or referring patients to another practice with an infusion center (6%).
Getting Coverage
Whether they prescribe biologics earlier or later in the disease process, physicians have found that prescribing more biologics often results in more work for them and their staff. Because of the high cost, health plans typically require time-consuming preauthorization procedures. These can create hassles for prescribing physicians, involving spending time on the phone and allocating more office staff time to resolving medication coverage issues.
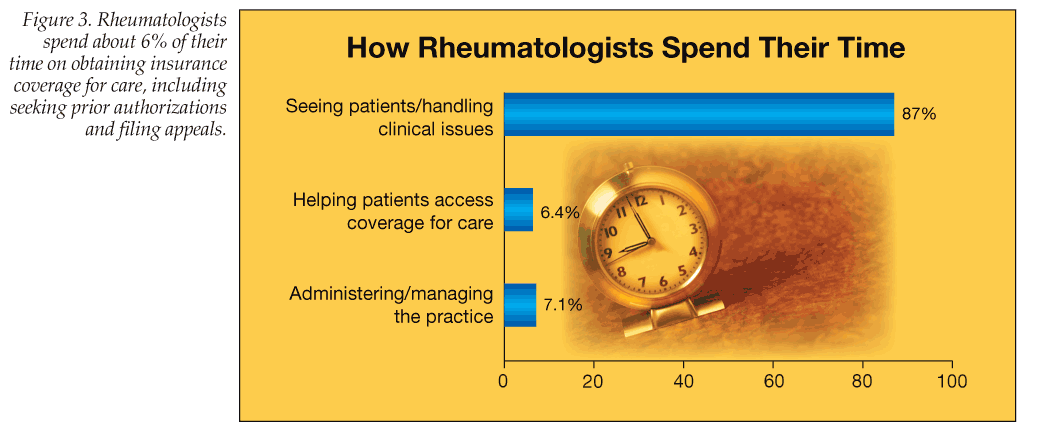
More than half of survey respondents (53.6%) say that insurers “often” try to restrict the use of biologics. Rheumatologists spent 6% of their time on average helping patients access coverage for care, such as obtaining authorizations and filing appeals (Figure 3). While 50% said billing and office staff had the primary responsibility for prior authorization and approval from insurers and 39.8% said a nurse had the primary responsibility, 7.1% said they personally had the primary responsibility for obtaining prior authorization and approval from health plans.
“Even though the office staff in many of our practices tends to do the bulk of it, we still have to spend a certain amount of time in the appeals process,” said Weiss. “That may be generating letters, reviewing charts, discussing treatment with patients, giving them the information. At least that is what I do.”
For staff with the responsibility for interacting with health plans, 45.4% of their time is spent on calls concerning coverage-related approval; 40% said they had to hire additional staff to handle payments and access to care, and 11.6% said they were planning to hire additional staff within the next year.
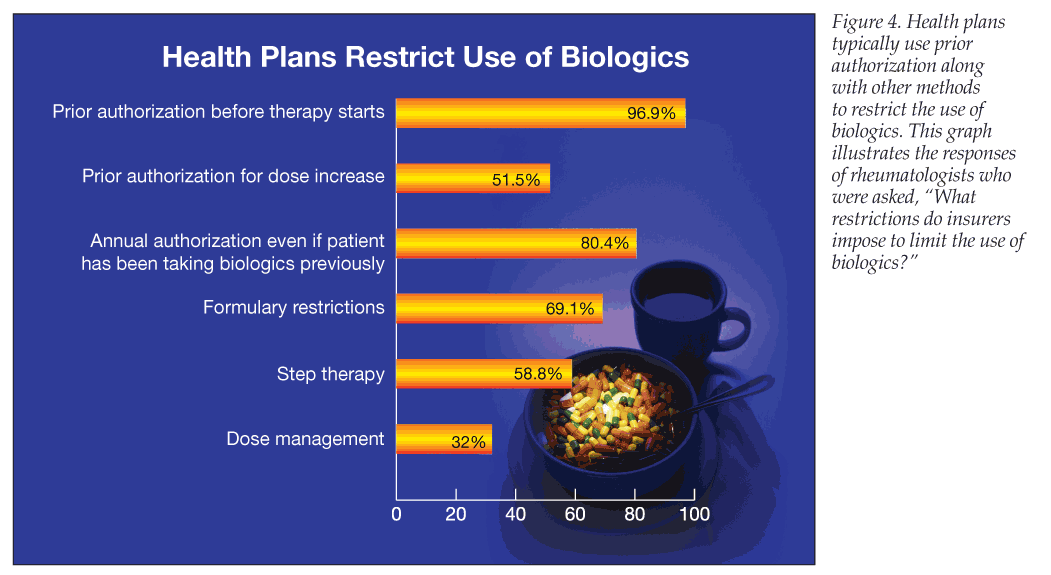
Almost all survey respondents acknowledged that restrictions on biologics are a problem: 53.6% said that health plans often try to restrict the use of biologics, 14.4% said they always do, and 19.6% said they usually do. The most common restriction cited by respondents was prior authorization before therapy starts (96.9%), followed by annual authorization, even if the patient has previously been taking biologics (80.4%) (Figure 4).
“Prior authorization-that’s where hell starts,” said Weiss. For prior authorization, doctors may be required to make phone calls, write letters, provide documentation concerning the patient’s disease and other medications they have used, he said. Such efforts by physicians and their staffs do not necessarily ensure approval.
Waiting for approval of a request can be frustrating as well. While 39.3% of respondents said they receive an answer from health plans about a prior authorization request in 2 to 4 days, others said they generally wait from a week (33%) to more than a month (1%). A prolonged approval process can be detrimental to patients for whom receiving a biologic agent early can make a difference in the course of their disease, said Weiss. “I have one patient right now who can’t get her medication and she had been off it for 4 to 6 weeks now.”
When a request for biologic therapy is denied, most physicians responding pursue an appeal. While 30% say their office staff can gain approvals through the health plan’s appeal process, often much of the work involved in appeals is done by the physician himself or herself-29.5% of respondents said they must speak to the health plan medical director; 18.9% said they must actively lobby the medical director with repeated letters and calls.
When patients do not have insurance to cover the agents, coverage is denied, or coinsurance amounts alone make the drugs unaffordable, physicians and patients often turn to patient assistance programs offered by pharmaceutical manufacturers. Most of the physicians responding were aware of programs offered by the manufacturers of adalimumab, etanercept, and infliximab. “Just about every one of these companies has done an excellent job,” said Weiss.
Conclusion
The use of biologics in rheumatology has changed the course of the disease for many patients and, despite the cost, most physicians plan to prescribe them for patients they believe will benefit from them-even with the additional time and effort it takes to get the drugs covered.
“Doctors have realized these are really good drugs and they are willing to spend some time themselves or hire people to get approval for these drugs,” said Waxman. “Even though it is a tough fight with insurance companies, we are recognizing that the patients do so well on these drugs that despite the hassles, they are willing to put up with it and go ahead with the new drugs.”
References:
References
1. Cohen S. Biologic therapies in the treatment of rheumatoid arthritis. US Musculoskeletal Review. 2006:92-93. http://www.touchbriefings.com/ cdps/cditem.cfm?NID=1857. Accessed July 20, 2009.
2. Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion. Rheumatoid arthritis. http://www.cdc.gov/arthritis/arthritis/ rheumatoid.htm. Accessed July 20, 2009.
3. UCB, Inc, National Association of Managed Care Physicians, Arthritis Foundation. Biologics Trend Report. Vol 1. http://biologicstrendreport.com. Accessed July 20, 2009.
