- Clinical Technology
- Adult Immunization
- Hepatology
- Pediatric Immunization
- Screening
- Psychiatry
- Allergy
- Women's Health
- Cardiology
- Pediatrics
- Dermatology
- Endocrinology
- Pain Management
- Gastroenterology
- Infectious Disease
- Obesity Medicine
- Rheumatology
- Nephrology
- Neurology
- Pulmonology
Minimally Invasive Esophageal String Test Used to Monitor Eosinophilic Esophagitis in Children
Researchers at Phoenix Children’s Hospital conducted one of the largest real-world clinical evaluations of the esophageal string test in children.
©Naeblys/AdobeStock
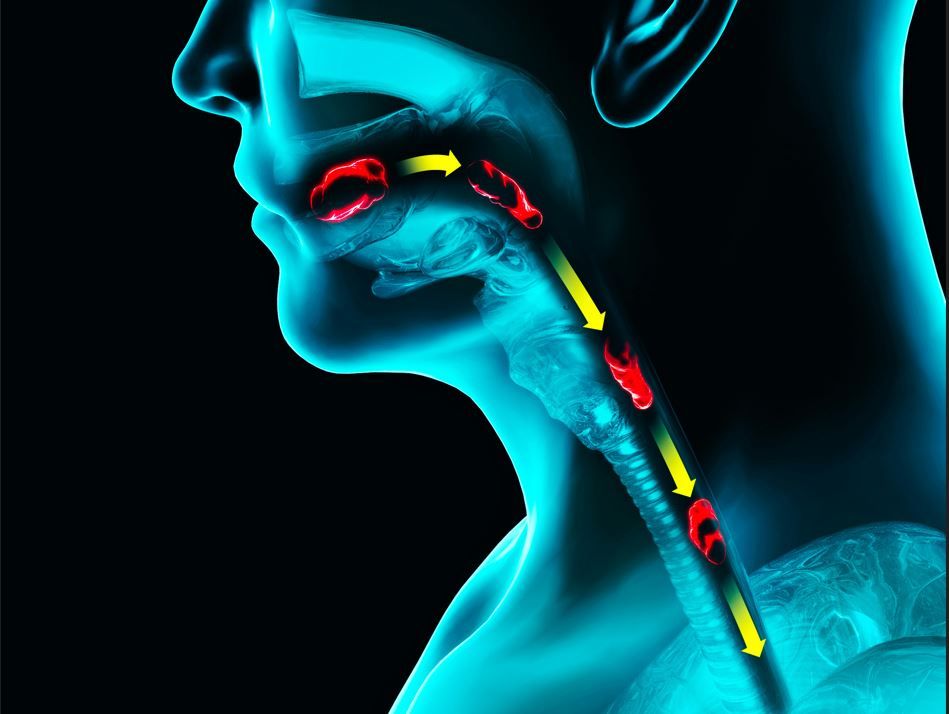
Physician-researchers at Phoenix Children’s have pioneered the use of the esophageal string test (EST) as a minimally invasive, sedation-free method for monitoring inflammation in children with eosinophilic esophagitis (EoE).1
The EST offers an alternative to traditional esophagogastroduodenoscopy (EGD) with biopsies, which require sedation. The EST involves swallowing a capsule containing a nylon string that collects esophageal secretions, providing biomarkers for disease assessment, according to a January 24 press release.1
EoE is a progressive inflammatory condition that can cause pain, difficulty swallowing, and esophageal damage. Standard monitoring methods require sedation, which increases procedure-related risks and time away from daily activities. The EST uses a swallowable capsule containing a nylon string to collect esophageal secretions, allowing for biomarker analysis without the need for anesthesia.1
“The esophageal string test is a significant step forward in improving the patient experience for children with eosinophilic esophagitis,” Shauna Schroeder, MD, co-medical director of Phoenix Children’s Eosinophilic Gastrointestinal Disease Clinic and lead author of the study, said in a news release.1 “This approach, when clinically indicated, spares children the discomfort and risks of repeated endoscopies under anesthesia, allowing us to monitor their condition in an outpatient clinic setting and make changes in their treatment.”
Most patients in the study tolerated the EST well, reporting only mild side effects such as sore throat. Study findings were published in The Journal of Allergy and Immunology: In Practice, and additional clinical practice recommendations from Phoenix Children’s researchers were published in The Journal of Allergy and Clinical Immunology.1
“This technology represents an opportunity to reimagine how we monitor and manage chronic esophageal diseases,” Cindy Bauer, MD, co-author of the study and division chief of allergy and immunology at Phoenix Children’s, said.1 “The ability to provide real-time, noninvasive monitoring opens doors for better disease management and improved outcomes.”
Phoenix Children’s researchers emphasize that the adoption of EST aligns with their goal of minimizing disruptions to patients’ daily lives while maintaining effective disease monitoring.1
“By offering an alternative tool that eliminates the need for sedation and reduces time away from school or work, patient families have a more accessible option for esophageal testing, and providers still collect necessary data for patient care,” co-author Benjamin Wright, MD, pediatric allergist and immunologist at Phoenix Children’s, said.1
There are currently 2 FDA approved medications for the treatment of EoE in children: dupilumab and budesonide. Dupilumab is the first and only FDA-approved treatment for EoE in children aged 1 and older and budesonide is the first oral therapy for EoE and is indicated for people aged 11 years and older.2,3
References:
1. Physician-researchers at Phoenix Children’s pioneer use of minimally invasive tool to monitor chronic esophageal disease in children. News release. Phoenix Children's Hospital. January 27, 2025. Accessed January 27, 2025. https://phoenixchildrens.org/articles-faqs/news-articles/phoenix-childrens-physician-researchers-pioneer-minimally-invasive-egid-tool
2. Halsey G. FDA approves dupilumab for eosinophilic esophagitis in children aged 1 to 11 years. Patient Care Online. January 26, 2024. https://www.patientcareonline.com/view/fda-approves-dupilumab-for-eosinophilic-esophagitis-in-children-aged-1-to-11-years
3. Halsey G. Takeda announces approval of first oral therapy for eosinophilic esophagitis. Patient Care Online. February 12, 2024. https://www.patientcareonline.com/view/takeda-announces-approval-of-first-oral-therapy-for-eosinophilic-esophagitis
Clinical Tips for Using Antibiotics and Corticosteroids in IBD
January 5th 2013The goals of therapy for patients with inflammatory bowel disorder include inducing and maintaining a steroid-free remission, preventing and treating the complications of the disease, minimizing treatment toxicity, achieving mucosal healing, and enhancing quality of life.
