- Clinical Technology
- Adult Immunization
- Hepatology
- Pediatric Immunization
- Screening
- Psychiatry
- Allergy
- Women's Health
- Cardiology
- Pediatrics
- Dermatology
- Endocrinology
- Pain Management
- Gastroenterology
- Infectious Disease
- Obesity Medicine
- Rheumatology
- Nephrology
- Neurology
- Pulmonology
Managing Allergic Rhinitis: The Role of Pharmacotherapy
While avoidance measures are a key component of the treatment of allergic rhinitis, pharmacological therapies are often needed to adequately control symptoms. Intranasal corticosteroids are highly effective and are particularly useful in patients with moderate to severe disease.
Since approximately 50 million Americans have allergic rhinitis (AR), almost all primary care physicians will encounter this disease.1 The prevalence of AR has increased dramatically in the past 30 years and may still be rising.2 The financial impact is significant, with the direct costs estimated to be $7.3 billion and indirect costs estimated at $4.28 billion, including loss of productivity. This amounts to a total cost of $11.58 billion in the United States for 2002.3
Patients with AR may experience a variety of symptoms, including sneezing, rhinorrhea, nasal itching, congestion, and/or postnasal drainage. Although it may be easy to dismiss this disease as innocuous, patients often experience headaches, fatigue, impaired concentration, reduced productivity, loss of sleep, increased occupational risks, and decreased emotional well-being and social functioning.
In treating patients with AR, the most important first step in achieving symptomatic improvement is avoidance of the offending allergen(s). If a patient has seasonal AR and is mainly sensitive to outdoor pollens, recognizing pollen seasons and remaining indoors during that time is most helpful. For dust mite allergy, allergen-proof covers for mattresses and pillows, frequent washing of linens in hot water, and eliminating carpeting are the first steps to successful management. For pet allergy, removal of the pet from the home is obviously important; however, this recommendation is rarely followed.
Avoidance measures are the mainstay of treatment. However, if such measures fail or are unrealistic, there are several pharmacological therapies that can successfully control the symptoms of AR. In this article, we review the benefits and limitations of the available pharmacological therapies.
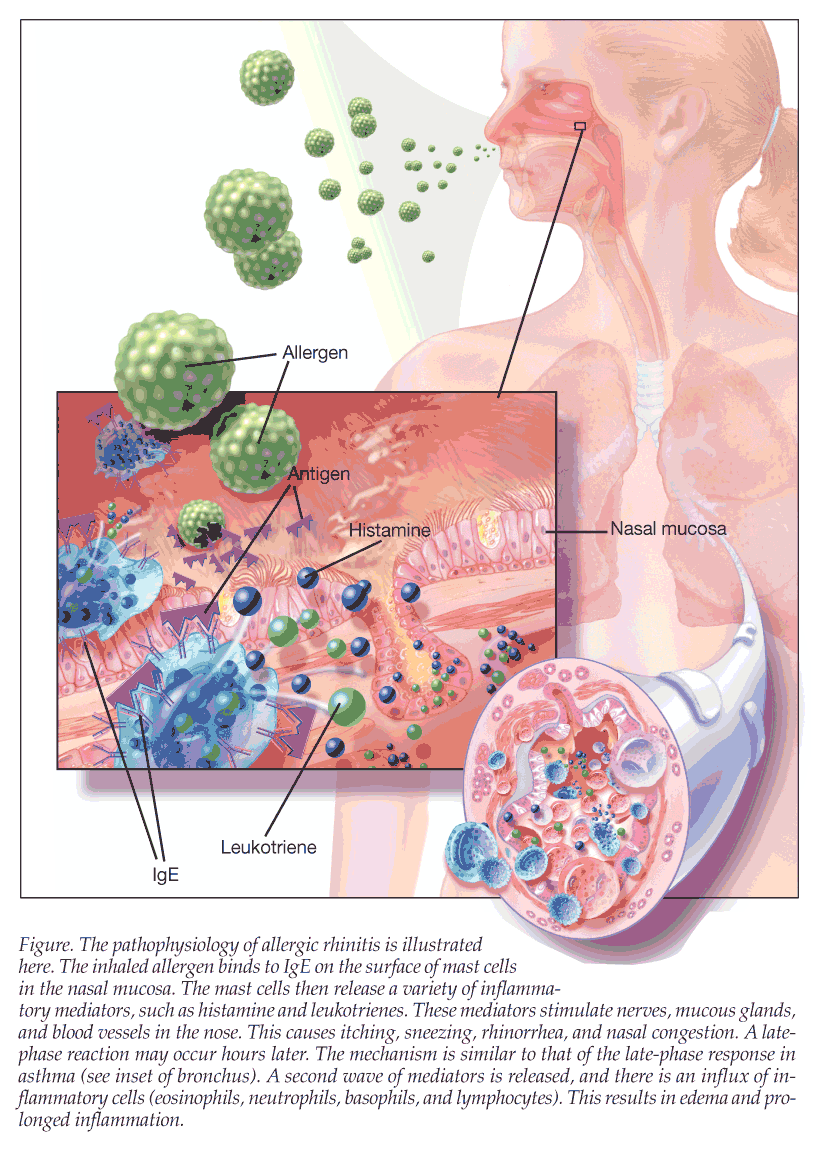
Understanding AR
In 2008, the Joint Task Force on Practice Parameters in Allergy, Asthma, and Immunology defined rhinitis as “characterized by one or more of the following nasal symptoms: congestion, rhinorrhea (anterior and posterior), sneezing, and itching.”4 AR, in particular, is a hypersensitivity reaction to specific allergens that occurs in sensitized patients, which is mediated by IgE antibodies that result in the inflammation.5 AR has been traditionally described as seasonal or perennial depending on the specific allergens to which the person is sensitized. The Joint Task Force also defines episodic allergic rhinitis as “allergic nasal symptoms elicited by sporadic exposures to inhalant aeroallergens that are not usually encountered in the patient’s indoor or outdoor environment, such as while visiting a home with pets when a patient has no usual exposure to pets.”4
This allergic reaction is typically divided into a 2-phase response: an early phase and a late phase. The early phase of AR occurs within minutes of allergen exposure and is characterized by sneezing, pruritus, rhinorrhea, and nasal congestion. It is produced by activation of tissue mast cells sensitized by IgE antibodies (Figure).
Approximately half of all patients with AR experience the late-phase response, which is typically characterized by congestion, about 4 to 12 hours after exposure.5 This phase corresponds with the recruitment of many inflammatory cells, including T lymphocytes, monocytes/macrophages, neutrophils, eosinophils, and basophils. AR also can be divided into seasonal, perennial, or episodic disease, depending on specific allergen sensitivities.
Unfortunately, the symptoms of AR often extend beyond the nose. Patients with AR frequently experience headaches, fatigue, impaired concentration, reduced productivity, loss of sleep, a decrease in emotional well-being and social functioning, and increased occupational risks.6,7 In fact, in patients with perennial symptoms, the degree of impairment of health-related quality of life is similar to that seen in patients with asthma.8
When AR is untreated or inadequately treated, symptoms may become chronic and contribute to associated conditions, such as rhinosinusitis, asthma, and/or otitis media. Many medical interventions have been shown to improve health-related quality of life in patients with AR and to prevent worsening of associated conditions.8
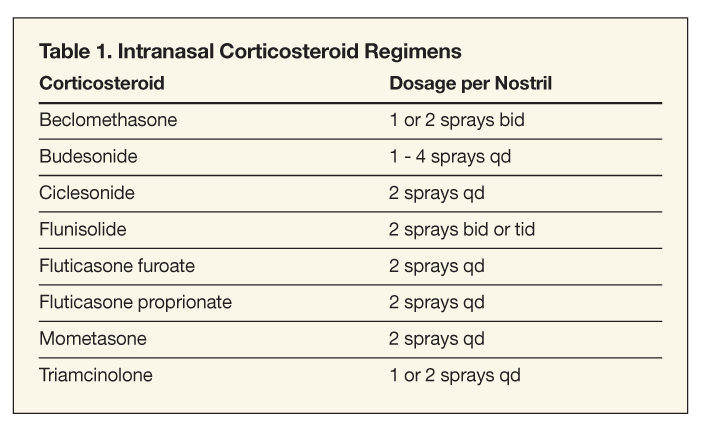
Intranasal Corticosteroids
These agents are the most effective medications in treating patients with AR and are increasingly becoming the mainstay of treatment.9,10 A number of preparations are available (Table 1). These medications provide effective relief for the complex of nasal symptoms associated with AR, as well as relief from congestion and even ocular symptoms to a certain extent.2 Despite earlier beliefs that intranasal corticosteroids must be used for at least 1 to 2 weeks before symptomatic improvement is seen, many reports have demonstrated clinical improvement within the first day.2,11,12 In fact, the onset of therapeutic effect has been found to occur between 3 and 12 hours.12-14
Intranasal corticosteroids are a highly effective group of medications for the treatment of AR, with efficacy exceeding that of antihistamines, decongestants, and cromolyn.15 Also, they are more cost-effective than oral antihistamines.2 Several studies have demonstrated that the efficacy of intranasal cortico- steroids is superior to that of the combined use of an antihistamine and leukotriene receptor antagonist (LTRA).16-20
Intranasal corticosteroids act by controlling the rate of protein synthesis, thereby inhibiting the release of mediators by inflammatory cells. This leads to vasoconstriction, which reduces edema and decreases influx of inflammatory cells into the nasal mucosa.15,21 Despite differences in lipid solubility, bioavailability, potency, and preparation (aqueous vs aerosol), there do not appear to be any clinically significant differences in efficacy among the available intranasal corticosteroids.11
Local adverse effects are relatively uncommon, with about 10% of patients reporting nasal irritation, nasal burning, or sneezing after administration. Approximately 2% report bloody nasal discharge, and there are a few case reports of septal perforations and delayed hypersensitivity reactions.15 It is important to emphasize that local adverse effects are rare and can be avoided with proper administration technique.
Systemic adverse effects are not considered a serious risk with intranasal corticosteroids.13 Studies have failed to show significant effects on serum markers of bone metabolism or cortisol concentrations after stimulation with adrenocorticotropic hormone.2 Further, there are no reports of acute adrenal crisis or chronic adrenal insufficiency occurring with the use of intranasal corticosteroids.11 Fluticasone proprionate and mometasone, which have been available for several years, and newer agents, including fluticasone furoate and ciclesonide, have systemic bioavailabilities below 1% and should be less likely than their predecessors to promote systemic effects.10 Ciclesonide is unique in that it is administered as a parent compound, and once it comes into contact with nasal epithelial esterases, it is cleaved into an active form, which has anti-inflammatory properties.
Many practitioners are concerned about prescribing intranasal corticosteroids for children, because of the bone growth suppression that is a potential adverse effect of oral corticosteroids. Growth suppression has been associated only with long-term use of beclomethasone at higher than recommended doses.22 The newer and more potent corticosteroids, such as mometasone, have not had these short-term effects on bone growth velocity.23
Topical corticosteroids are generally well tolerated by elderly patients, with minimal adverse effects.24 Intranasal budesonide is labeled as pregnancy category B for the treatment of AR.
Central to treatment efficacy with intranasal corticosteroids is patient education. Adherence is improved when proper insufflation technique is demonstrated. It is imperative to emphasize that doses be directed away from the septum and that once-daily medications be administered in the morning. After 1 to 2 weeks of treatment, the patient should be reevaluated and the dose adjusted on the basis of the response to therapy, with the goal being symptom relief at the lowest effective dose.15
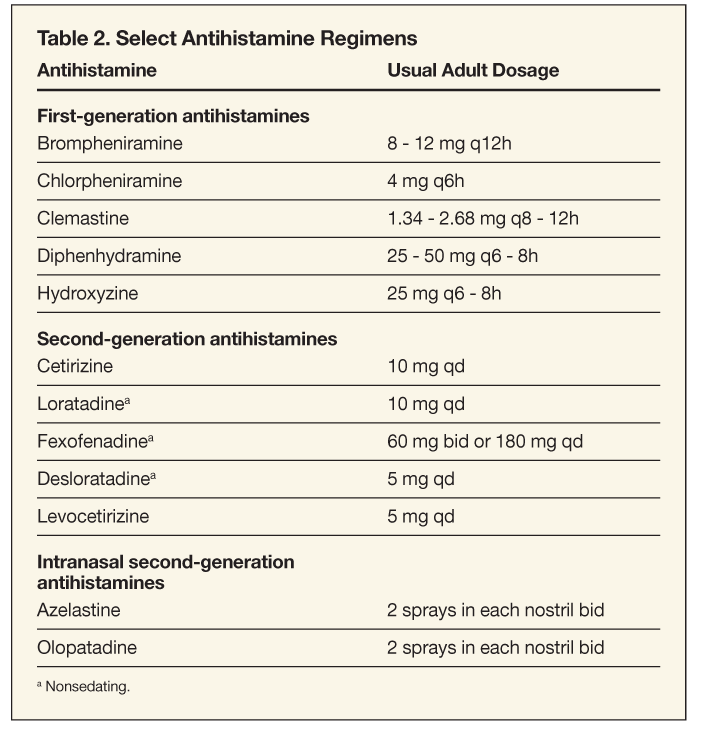
Antihistamines
These are the traditional drugs of choice for AR and are still considered first-line therapy for mild disease. Antihistamines may be given alone or in combination with intranasal corticosteroids, and they control symptoms of sneezing, nasal itching, rhinorrhea, and conjunctival itching and redness. However, antihistamines are generally not fully adequate for treatment of nasal congestion, although some of the newer agents do have decongestant effects. A number of first-generation and second-generation agents are available, in oral and topical form (Table 2).
Many of the symptoms of AR are directly related to the release of histamine from inflammatory cells and subsequent ligation to the H1 receptor. Antihistamines work by competitive inhibition at the receptor level.
First-generation antihistamines. These anti- histamines were the first group of medications available to treat the symptoms of AR. Although first-generation antihistamines are readily available in OTC preparations, it is prudent to avoid their use, if possible, because of their adverse-effect profiles. These medications are efficient in blocking the H1 receptor; however, their specificity for the H1 receptor is quite low. First-generation antihistamines bind to anticholinergic, serotonergic, and a-adrenergic receptors as well, burdening patients with many untoward effects, since these molecules cross freely through the blood-brain barrier.6
Adverse effects of first-generation antihistamines include blurry vision, dry eyes, constipation, urinary retention, bradycardia, drowsiness, decreased alertness, somnolence, restlessness, and insomnia.6 Further, they have been shown to impair performance by affecting sustained attention, cerebral processing, visual function, and reaction time.25 Although the product packaging warns of the sedative effects and warns against operating heavy machinery or driving motor vehicles, these warnings are often overlooked. It is important to educate patients taking first-generation antihistamines about the direct effect these medications have on vigilance and concentration.
Weiler and associates26 showed that driving response time was reduced and steering instability was significantly greater in patients who had taken 50 mg of diphenhydra- mine compared with placebo. In fact, the diphenhydramine group had greater steering instability than the group who had imbibed alcohol to reach a blood alcohol concentration of approximately 0.1%. It is recognized in 35 states and the District of Columbia that these medications can impair driving performance, and it is prohibited by law to operate a motor vehicle while under the influence of agents that can impair driving performance.25
Consequently, first-generation antihistamines must be avoided in patients who pilot planes, drive extensively, or operate heavy or dangerous machinery. In addition, patients who have preexisting intellectual impairment, elevated intraocular pressure, or benign prostatic hypertrophy or other forms of bladder outlet obstruction are at greater risk for adverse effects from this generation of antihistamines.4
It has been erroneously suggested that the soporific adverse effects of first-generation antihistamines may be managed by taking medications before bedtime. However, studies prove that this strategy leads to drowsiness and decreased concentration the following day.25 The decrease in productivity secondary to these adverse effects clearly negates any perceived cost benefit when compared with second-generation medications.
Tolerance to these adverse effects can develop, but such tolerance is not consistent, universal, or predictable. Patients must be notified that tolerance only occurs on regular dosing and that taking the medication on an as-needed or intermittent basis will continue to cause sedative effects.25
As a general rule, first-generation antihistamines must be avoided by elderly patients.24 However, in pregnant women, agents in this group, especially chlorpheniramine, are favored over newer antihistamines because of their shorter plasma half-life and long history of safe administration during pregnancy.27,28 However, their sedative qualities may make them less desirable choices.4
Second-generation antihistamines. These antihistamines have been designed to have less lipophilicity and are often larger molecules than their first-generation predecessors. In addition, several second-generation antihistamines possess a different ionic charge than first-generation antihistamines. Thus, these newer medications are less likely to cross the blood-brain barrier, leading to minimal sedation or psychomotor impairment.10,21
Notably, these newer antihistamines are generally free of anticholinergic effects and adverse GI effects. The rapid onset and extended duration of action, often up to 24 hours, make this group of medications particularly desirable.6
Two medications in this category, astemizole and terfenadine, were voluntarily withdrawn from the North American market because of cardiac adverse effects. Serum accumulation of these medications was associated with prolongation of the QT interval. When serum levels were extremely elevated, there was a risk of ventricular arrhythmias, cardiac arrest, or sudden cardiac death.21 These cardiac events have not been associated with currently available antihistamines.
Cetirizine, loratadine, and fexofenadine all have been shown to be more effective than placebo for treatment of AR. Cetirizine has slightly more sedative effects than the others and should generally be taken at night, but it has also been shown to be the most potent of the 3 by several cutaneous inhibition studies.29 Loratadine and cetirizine are both recognized as pregnancy category B.
Desloratadine is a newer second-generation antihistamine and is the active metabolite of loratadine. It has been shown to have rapid onset of action, sustained efficacy, and anti-inflammatory properties. Desloratadine also appears to have a mild beneficial effect on nasal congestion. Studies of desloratadine have not shown adverse effects on the CNS or the cardiovascular, renal, and hepatic systems, and this agent requires no dosing adjustment based on age.1
Levocetirizine, the active isomer of its parent compound, cetirizine, is one of the newest second-generation antihistamines. After only 1 dose, it has been found to suppress the cutaneous allergic response to a significantly greater extent than similar drugs in its class.29 In addition, levocetirizine is effective in the treatment of nasal congestion.30 Levocetirizine may be associated with a slightly higher risk of sedation, although this effect may be less than that observed with cetirizine.10
Intranasal second-generation antihistamines. Intranasal azelastine and olopatadine are the cur- rently available topical second-generation antihistamines. Intranasal antihistamines have demonstrated efficacy equivalent to that of oral second-generation antihistamines; however, they are not as effective as intranasal corticosteroids for treatment of AR.31 Both azelastine and olopatadine have a rapid onset of action (minutes), and these effects can last up to 12 hours. However, bitter taste has been reported with both preparations, and sedation may occur. To counteract the bitter aftertaste, a newer formulation of azelastine contains sorbitol and sucralose.
Decongestants
Oral decongestants. Phenylpropanolamine, phenylephrine, and pseudoephedrine are decongestants that affect vasoconstrictor activity by releasing noradrenaline from sympathetic nerve endings and directly stimulate adrenoreceptors in blood vessels in the nasal mucosa, which helps relieve nasal congestion. Phenylephrine is the most widely available decongestant in the United States at this time. Phenylpropanolamine is no longer marketed in the United States, and use of pseudo- ephedrine is restricted because of its use in the illegal manufacturing of methamphetamine.
Decongestants may have a mild effect in treating rhinorrhea but are not helpful in treating nasal itching, sneezing, or ocular symptoms; consequently, these medications are often given in combination with antihistamines.21 There are commercially available combinations of the antihistamines loratadine, cetirizine, and fexofenadine with the decongestant pseudoephedrine.
The most common adverse effects of decongestants include tachycardia, palpitations, headache, dizziness, nausea, and insomnia. Sympathomimetics should be used with caution in patients with hypertension, diabetes mellitus, ischemic heart disease, increased intraocular pressure, hyperthyroidism, or pros- tatic hypertrophy. In pregnancy, oral decongestants should be avoided in the first trimester because of the possible risk of gastroschisis.4
Intranasal decongestants. Intranasal decongestants (such as oxymetazoline and xylometazoline) are available in OTC preparations. These may be useful during the initiation of therapy with intranasal corticosteroids-by first opening the nasal passages with an intranasal decongestant, then insufflating the corticosteroid, which allows for better distribution of the latter.
Patients should be warned that overuse of these medications may cause rebound congestion, nasal hyperreactivity, tolerance, and changes in the nasal mucosa (rhinitis medicamentosa).32 Use of these medications usually should be limited to 3 to 5 days.
Because of the extensive adverse-effect profile of these medications and the likelihood that elderly patients are more susceptible to adverse effects, the use of decongestants should be carefully considered in this population.4
Cromolyn
This agent inhibits the degranulation of sensitized mast cells, which occurs after exposure to specific antigens/ allergens. Cromolyn has only mild anti-inflammatory activity, and it has no intrinsic antihistaminic activity. Sneezing, nasal itching, and rhinorrhea respond well to cromolyn, but this agent is considered ineffective treatment for nasal congestion. Intranasal cromolyn is available without a prescription.
The most frequent adverse reactions associated with cromolyn are sneezing and nasal stinging, burning, and irritation. Headaches and a bad taste in the mouth are reported in about 2% of patients. Epistaxis, postnasal drip, and rash are reported in fewer than 1%. There have been case reports of anaphylaxis attributable to cromolyn.
The maximum benefit with cromolyn occurs approximately 2 to 4 weeks after initiation of therapy. For the treatment of seasonal symptoms, it is advisable to begin therapy 2 weeks before expected allergen exposure. Cromolyn given 4 times a day is as effective as antihistamine therapy but is generally considered less effective than intranasal corticosteroids; it may be given up to 6 times a day.21 Unfortunately, this frequent dosing schedule makes adherence a challenge.
Cromolyn is safe and effective therapy for elderly patients and pregnant women.4,24
Ipratropium
This anticholinergic agent is available as a 0.03% and 0.06% intranasal solution, and both formulations have indications for the treatment of AR. Ipratropium significantly and rapidly reduces secretions associated with AR, without excessive dryness or potential cholinergic adverse effects.33 It is indicated for use in patients who complain of rhinorrhea as their main symptom.34
Ipratropium has very little effect on other symptoms of AR. Common adverse effects include nasal irritation/crusting and occasional mild epistaxis. The regimen for ipratropium is 2 sprays per nostril 2 to 4 times a day.
Leukotriene Receptor Antagonists
Zafirlukast and montelukast are selective and competitive receptor antagonists of leukotriene D4 and E4. No significant difference in efficacy has been found between LTRA and antihistamines, and the effects of the medications when used together may be additive,18,35,36 although monotherapy with an intranasal corticosteroid is still superior.16-20 However, for those who may have difficulty with adherence to intranasal corticosteroids, combination oral therapy with an LTRA and an antihistamine may be a viable option.4 Also, since a large portion of patients with asthma have concurrent AR, the addition of an LTRA, such as montelukast, may be advantageous.4
Monoclonal Anti-IgE Antibody
The monoclonal anti-IgE antibody omalizumab is a recombinant humanized antibody that binds to the Fc portion of human IgE, inhibiting the binding of IgE to its receptor. Casale and associates37 showed that omalizumab not only decreases se- rum IgE levels but also provides symptomatic relief in patients with AR and improves patients’ quality of life in a dose-dependent manner, with minimal adverse effects. However, superiority to other available treatments has not been demonstrated. This, in combination with the high cost of omalizumab, precludes its use in patients with AR who do not have concurrent aeroallergen- associated asthma.4
Immunotherapy
At times, allergen avoidance and pharmacotherapy are not enough to provide sufficient relief for those with AR. Some patients cannot tolerate the adverse effects of medications, or they prefer long-term symptom control. For these patients, immunotherapy is an option.
Conclusion
For most patients who have AR, avoidance of the inciting allergen and appropriate use of pharmaco- therapy will bring symptomatic relief and restore quality of life. Finding a medication regimen that is suitable for the patient requires manipulating regimens in a stepwise fashion according to the patient’s disease severity and symptom complex. It is prudent to remember adverse-effect profiles, costs, and risks of drug interactions when prescribing any of the medications used to treat AR. Patients should be routinely monitored for adverse effects and therapeutic response.
References:
References
1. Geha RS, Meltzer EO. Desloratadine: a new, nonsedating, oral antihistamine. J Allergy Clin Immunol. 2001;107:751-762.
2. Weiner JM, Abramson MJ, Puy RM. Intranasal corticosteroids versus oral H1 receptor antagonists in allergic rhinitis: systematic review of randomized controlled trials. BMJ. 1998;317:1624-1629.
3. Schoenwetter WF, Dupclay L Jr, Appajosyula S, et al. Economic impact and quality-of-life burden of allergic rhinitis. Curr Med Res Opin. 2004; 20:305-317.
4. Wallace DV, Dykewicz MS, Bernstein DI, et al; Joint Task Force on Practice; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. The diagnosis and management of rhinitis: an updated practice parameter [published correction appears in J Allergy Clin Immunol. 2008; 122:1237]. J Allergy Clin Immunol. 2008;122 (2 suppl):S1-S84.
5. Naclerio R. Clinical manifestations of the release of histamine and other inflammatory mediators. J Allergy Clin Immunol. 1999;103(3, pt 2):S382-S385.
6. Prenner BM. The evolution of pharmacotherapy for rhinitis and urticaria. Allergy Asthma Proc. 2001;22:277-280.
7. Thompson AK, Juniper E, Meltzer EO. Quality of life in patients with allergic rhinitis. Ann Allergy Asthma Immunol. 2000;85:338-347.
8. Meltzer EO. Quality of life in adults and children with allergic rhinitis. J Allergy Clin Immunol. 2001; 108(1 suppl):S45-S53.
9. Van Cauwenberge P, Bachert C, Passalacqua G, et al. Consensus statement on the treatment of allergic rhinitis. European Academy of Allergology and Clinical Immunology. Allergy. 2000;55: 116-134.
10. Krouse JH. Allergic rhinitis-current pharmacotherapy. Otolaryngol Clin North Am. 2008;41: 347-358.
11. Corren J. Intranasal corticosteroids for allergic rhinitis: how do different agents compare? J Allergy Clin Immunol. 1999;104(4, pt 1):S144-S149.
12. Day JH, Briscoe MP, Rafeiro E, et al. Onset of action of intranasal budesonide (Rhinocort aqua) in seasonal allergic rhinitis in a controlled exposure model. J Allergy Clin Immunol. 2000;105:489-494.
13. Meltzer EO, Rickard KA, Westlund RE, Cook CK. Onset of therapeutic effect of fluticasone propionate aqueous nasal spray. Ann Allergy Asthma Immunol. 2001;86:286-291.
14. Berkowitz RB, Bernstein DI, LaForce C, et al. Onset of action of mometasone furoate nasal spray (NASONEX) in seasonal allergic rhinitis. Allergy. 1999;54:64-69.
15. LaForce C. Use of nasal steroids in managing allergic rhinitis. J Allergy Clin Immunol. 1999;103 (3, pt 2):S388-S394.
16. Pullerits T, Praks L, Ristioja V, Lötvall J. Comparison of a nasal glucocorticoid, antileuko- triene, and a combination of antileukotriene and antihistamine in the treatment of seasonal allergic rhinitis. J Allergy Clin Immunol. 2002; 109:949-955. 1
7. Di Lorenzo G, Pacor ML, Pellitteri ME, et al. Randomized placebo-controlled trial comparing fluticasone aqueous nasal spray in mono-therapy, fluticasone plus cetirizine, fluticasone plus montelukast and cetirizine plus montelukast for seasonal allergic rhinitis [published correction appears in Clin Exp Allergy. 2004;34:1329]. Clin Exp Allergy. 2004;34:259-267.
18. Wilson AM, O’Byrne PM, Parameswaran K. Leukotriene receptor antagonists for allergic rhinitis: a systematic review and meta-analysis. Am J Med. 2004;116:338-344.
19. Wilson AM, Orr LC, Sims EJ, et al. Antiasthmatic effects of mediator blockade versus topical corticosteroids in allergic rhinitis and asthma. Am J Respir Crit Care Med. 2000;162(4 pt 1):1297-1301.
20. Wilson AM, Orr LC, Sims EJ, Lipworth BJ. Effects of monotherapy with intra-nasal cortico- steroid or combined oral histamine and leuko-triene receptor antagonists in seasonal allergic rhinitis. Clin Exp Allergy. 2001;31:61-68.
21. Corren J. Allergic rhinitis: treating the adult. J Allergy Clin Immunol. 2000;105(6, pt 2):S610-S615.
22. Schenkel EJ, Skoner DP, Bronsky EA, et al. Absence of growth retardation in children with perennial allergic rhinitis after one year of treatment with mometasone furoate aqueous nasal spray. Pediatrics. 2000;105:E22.
23. Pedersen S. Assessing the effect of intranasal steroids on growth. J Allergy Clin Immunol. 2001; 108(1 suppl):S40-S44.
24. Tan R, Corren J. Optimum treatment of rhinitis in the elderly. Drugs Aging. 1995;7:168-175.
25. Nolen TM. Sedative effects of antihistamines: safety, performance, learning, and quality of life. Clin Ther. 1997;19:39-55.
26. Weiler JM, Bloomfield JR, Woodworth GG, et al. Effects of fexofenadine, diphenhydramine, and alcohol on driving performance. A randomized, placebo-controlled trial in the Iowa driving simulator. Ann Intern Med. 2000;132:354-363.
27. Seto A, Einarson T, Koren G. Pregnancy outcome following first trimester exposure to antihistamines: meta-analysis. Am J Perinatol. 1997;14:119-124.
28. Grant JA. Molecular pharmacology of second-generation antihistamines. Allergy Asthma Proc. 2000;21:135-140.
29. Grant JA, Riethuisen JM, Moulaert B, DeVos C. A double-blind, randomized, single-dose, cross-over comparison of levocetirizine with ebastine, fexofenadine, loratadine, mizolastine, and placebo: suppression of histamine-induced wheal-and-flare response during 24 hours in healthy male subjects. Ann Allergy Asthma Immunol. 2002; 88:190-197.
30. Bachert C, Bousquet J, Canonica GW, et al; XPERT Study Group. Levocetirizine improves quality of life and reduces costs in long-term management of persistent allergic rhinitis. J Allergy Clin Immunol. 2004;114:838-844.
31. Yanez A, Rodrigo GJ. Intranasal corticosteroids versus topical H1 receptor antagonists for the treatment of allergic rhinitis: a systematic review with meta-analysis. Ann Allergy Asthma Immunol. 2002;89:479-484.
32. Graf P. Rhinitis medicamentosa: aspects of pathophysiology and treatment. Allergy. 1997;52 (40 suppl):28-34.
33. Kaiser HB, Findlay SR, Georgitis JW, et al. The anticholinergic agent, ipratropium bromide, is useful in the treatment of rhinorrhea associated with perennial allergic rhinitis. Allergy Asthma Proc. 1998;19:23-29.
34. Dockhorn R, Aaronson D, Bronsky E, et al. Ipratropium bromide nasal spray 0.03% and beclomethasone nasal spray alone and in combination for the treatment of rhinorrhea in perennial rhinitis. Ann Allergy Asthma Immunol. 1999; 82:349-359.
35. van Adelsberg J, Philip G, Pedinoff AJ, et al; Montelukast Fall Rhinitis Study Group. Montelukast improves symptoms of seasonal allergic rhinitis over a 4-week treatment period [published corrections appear in Allergy. 2004;59:357; Allergy. 2009;64:1697]. Allergy. 2003;58:1268-1276.
36. Philip G, Malmstrom K, Hampel FC, et al; Montelukast Spring Rhinitis Study Group. Montelukast for treating seasonal allergic rhinitis: a randomized, double-blind, placebo-controlled trial performed in the spring. Clin Exp Allergy. 2002;32:1020-1028.
37. Casale T, Condemi J, LaForce C, et al; Omalizumab Seasonal Allergic Rhinitis Trial Group. Effect of omalizumab on symptoms of seasonal allergic rhinitis: a randomized controlled trial. JAMA. 2001;286:2956-2967.
