- Clinical Technology
- Adult Immunization
- Hepatology
- Pediatric Immunization
- Screening
- Psychiatry
- Allergy
- Women's Health
- Cardiology
- Pediatrics
- Dermatology
- Endocrinology
- Pain Management
- Gastroenterology
- Infectious Disease
- Obesity Medicine
- Rheumatology
- Nephrology
- Neurology
- Pulmonology
Insomnia: Recent Advances in Pharmacological Management
The medications currently approved for the treatment of insomnia include 9 benzodiazepine receptor agonist (BZRA) hypnotics and the selective melatonin receptor agonist ramelteon.
The pharmacological management of insomnia has a very long history.1 It seems likely that fermented beverages and sedating plant concoctions (such as opium) have been used for millennia for their sleep-promoting effects. With patent medicines came products such as laudanum, which frequently were taken to combat insomnia. This was followed by chloral hydrate in the 1860s and barbiturates in the early 1900s.
Several sedating pharmaceuticals, such as methyprylon, glutethimide, ethchlorvynol, methaqualone, and meprobamate, transiently were popular in the mid-1900s. Although all of these may facilitate sleep, they also are associated with major safety concerns, including toxic effects that may be fatal.
Beginning in the 1960s and 1970s, benzodiazepines and related hypnotics became the most widely used insomnia medications. Recent pharmacological innovations in the treatment of insomnia have includ-ed extended-release and alternate-delivery formulations of existing hypnotics, as well as compounds with entirely new mechanisms of action. Throughout this long history of insomnia treatment, efficacy in promoting sleep has not been the primary challenge. Rather, it has been enhancing sleep safely while avoiding undesired daytime sedation or impairment.
In June 2005, the NIH sponsored the State-of-the-Science Conference on the Manifestations and Management of Chronic Insomnia, which included presentations on the characteristics, causes, and consequences of insomnia, as well as an evidence-based review of the literature on treatment.2 The summary report from the conference panel noted the well-established benefits of cognitive-behavioral therapy and the FDA-approved hypnotics in treating chronic insomnia, but they did not endorse the use of OTC antihistamine sleep aids or sedating antidepressants and antipsychotics because of the lack of efficacy data or the presence of significant safety concerns.2 The report called for longer-term studies of the pharmacological treatment of insomnia that better represented clinical practice and that assessed a broader range of effectiveness outcomes, particularly daytime functioning and the course of comorbid conditions.
One significant recent advance in the prescribing guidelines for insomnia medications relates to the recommended duration of use. The FDA indications for treatment previously specified “short-term” use. Beginning in 2005, the medications approved for the treatment of insomnia have included no implied limitation on duration of use. Long-term safety and efficacy studies have been performed with all of the recently approved medications, including eszopiclone,3,4 zolpidem extended- release,5 and ramelteon.6 All have demonstrated continued efficacy without the development of tolerance, as well as generally positive safety profiles.
Another relatively recent prescribing guideline development has been the specification of an indication for the treatment of sleep maintenance apart from the sleep onset symptoms, which should improve with any of the current insomnia medications.
This article will review the recent advances with the insomnia treatment medications that currently are approved by the FDA and then will discuss compounds that are being investigated as possible insomnia treatments.
Approved Therapies
In the United States, 9 benzodiazepine receptor agonist (BZRA) hypnotic formulations and 1 selective melatonin receptor agonist are approved by the FDA for the treatment of insomnia.7 These medications vary with regard to pharmacodynamic action and pharmacokinetic properties. No single medication is ideal for every insomnia patient-there is no “one size fits all” prescription for managing sleep disturbances. Patients vary in their insomnia symptoms, comorbid disorders, concurrent medications, demographic characteristics, and treatment preferences.
Benzodiazepine receptor agonist hypnotics. The BZRA hypnotics have been the primary pharmacological agents for the treatment of insomnia over the past 4 decades. This category constitutes the compounds incorporating the benzodiazepine structure that were formally indicated for insomnia treatment in the early 1970s and the nonbenzodiazepine agents that became available beginning in the 1990s. All share the same fundamental mechanism of action, although the newer nonbenzodiazepines have selective pharmacodynamic properties that may improve their safety and tolerability.7
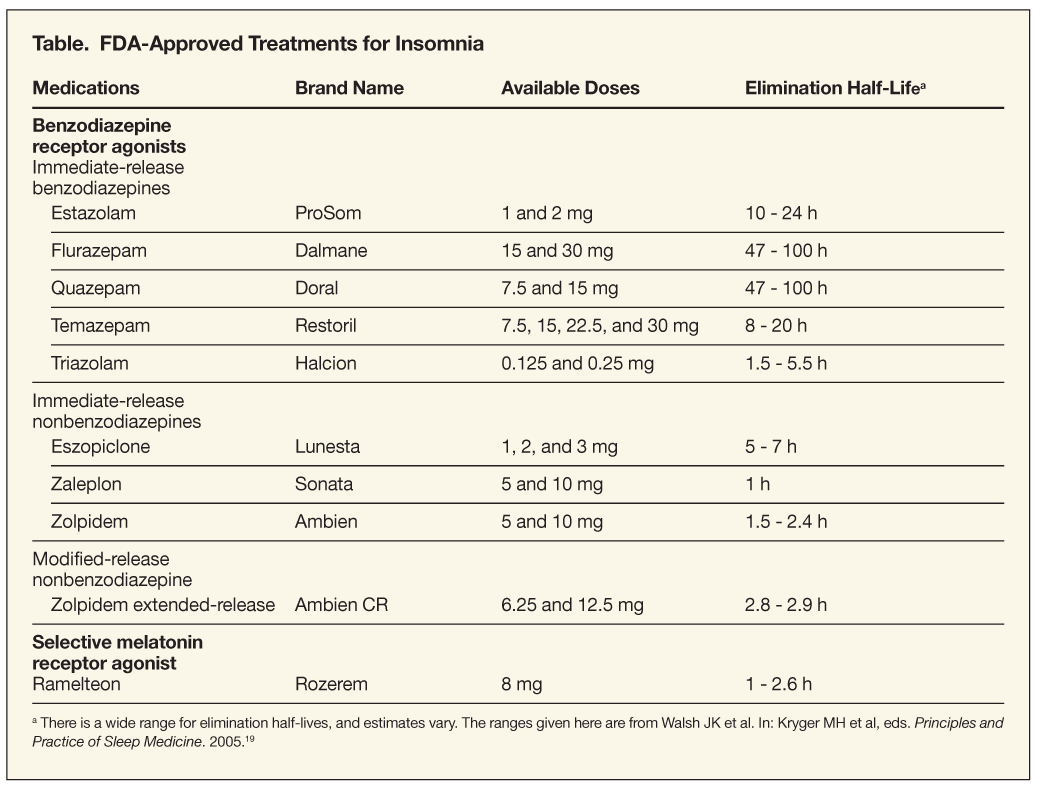
The 9 BZRA hypnotics that are FDA-approved for the treatment of insomnia are shown in the Table. All are Drug Enforcement Administration (DEA) Schedule IV controlled substances because they have a low potential for abuse and dependence.
The BZRA hypnotics are positive allosteric modulators of ϒ-aminobutyric acid (GABA) responses at the GABAA receptor complex.8 GABA is the most widespread inhibitory neurotransmitter in the CNS and plays a key role in the sleep-regulatory mechanisms in the hypothalamus. The GABAA receptor is a 5-subunit ligand-gated receptor with a central chloride ion channel. When GABA attaches at this receptor, extracellular chloride ions can flow through the cell membrane. This process creates an inhibitory effect by causing increased polarization and a decreased likelihood of an action potential occurring.
BZRAs interact with an allosteric recognition site on the GABAA receptor complex. The result is that more chloride ions can enter the cell and subsequently produce a greater degree of polarization that enhances the natural inhibitory action of GABA.
The newer-generation nonbenzodiazepine BZRA hypnotics lack the defining benzodiazepine structure. Compared with the benzodiazepine hypnotics, the nonbenzodiazepines tend to be more selective for GABAA receptor complexes containing an a1 subunit subtype.7 While several of the benzodiazepine hypnotics have rather long half-lives that may affect daytime functioning, the nonbenzodiazepine hypnotics tend to have considerably shorter half-lives and better target sedation exclusively during the nighttime.
The development of modified-release formulations of BZRA hypnotics has been a key pharmacokinetic innovation within this class. With the use of immediate-release formulations, the fundamental challenge is to provide a blood level of the hypnotic that is adequate to aid sleep maintenance during the night while avoiding undesired sedation or impairment the following morning. When an immediate-release medication with a short half-life does not provide sufficiently sustained action throughout the night, the use of a higher bedtime dose or an alternative with a long half-life is not an ideal solution.
The rationale for a modified- release formulation is that a compound with a relatively short half-life can be prepared in a pill form that allows both rapid and sus-tained release of the medication. The short half-life allows a relatively rapid decline later during the night to minimize the potential for next-day effects.9 The only modified- release hypnotic approved by the FDA is zolpidem extended-release,10 although other agents have been investigated as well.11
Selective melatonin receptor agonist. The approval of ramelteon in 2005 represented a major advance in the pharmacological treatment of insomnia because it was the first compound with an entirely new mechanism of action approved for this indication in 35 years. Ramelteon is a selective agonist for melatonin MT1 and MT2 receptor subtypes, which are most highly concentrated in the hypothalamic suprachiasmatic nucleus (SCN).12 The SCN functions as the master timekeeper of the circadian system. The timing of the sleep-wake cycle is influenced by the SCN, which is entrained by the photoperiod.
The circadian system helps maintain the waking state throughout the day and evening by promoting increasing arousal late in the daytime and into the evening. As bedtime approaches, the circadian arousal declines, leaving the homeostatic sleepiness that has accumulated since sleep last occurred unopposed. Endogenous melatonin has a central role in this process. Normally, the melatonin level is low throughout the daytime, but it gradually rises as bedtime approaches. Melatonin agonist activity decreases the firing rate of SCN neurons, which is thought to decrease circadian arousal. Under normal circumstances, this interaction of the circadian and homeostatic processes allows sleep onset to occur relatively rapidly at bedtime.
Ramelteon’s agonist activity at these SCN receptors enhances sleep onset by decreasing the evening circadian arousal. The approved indication is for the treatment of insomnia characterized by difficulty with sleep onset, which is consistent with the proposed mechanism of action. Ramelteon has been shown to improve sleep during the early portion of the sleep period. It also may reinforce the timing of the circadian system to increase the probability of sleepiness occurring regularly at bedtime. There is no implied limitation in the duration for which it can be prescribed. Ramelteon is classified as nonscheduled by the DEA because of an absence of abuse liability.13
Ramelteon is not a sedating medication, and patients may experience the maximum therapeutic effects over a period of several nights or weeks. Ramelteon is available in a single 8-mg dose. The typical recommendation is to take the medication about 30 minutes before bedtime. Although ramelteon is not associated with cognitive or psychomotor impairment, patients should avoid hazardous activities after taking it. Ramelteon should be avoided in patients with moderate to severe hepatic impairment and in persons concomitantly taking fluvoxamine. It has been associated with a low incidence of somnolence, fatigue, and dizziness.14
The variety of FDA-approved medications allows for customization in developing treatment strategies for patients, depending on their specific sleep-related symptoms, age, substance abuse history, and comorbid condition. Based on the pharmacokinetic properties and mechanism of action, it should be possible to select a medication with effectiveness in improving nighttime symptoms while avoiding undesired next-morning residual effects.
As noted, all of the approved medications have indications for helping with sleep-onset insomnia, but only certain hypnotics are beneficial for sleep maintenance. If substance abuse has been a major problem, a BZRA may not be the initial choice. Similarly, greater caution should be exercised in the medication selection for patients who are more vulnerable to the effects of ataxia or respiratory depression.
Investigational Compounds
Currently, a rich assortment of compounds is being investigated for the treatment of insomnia. Development of some of the molecules now being evaluated has been stimulated by the emerging knowledge of the neurophysiological regulation of sleep and wakefulness. Others have been developed as variations of the current pharmacological strategies for medications approved for the treatment of insomnia or used on an off-label basis. Among these are compounds in early investigational studies, but others have completed phase 3 studies and, if approved by the FDA, could be marketed within 1 to 2 years.
Two new compounds that had been studied extensively for the treatment of insomnia, but which now apparently have been abandoned, are gaboxadol and indiplon. Further studies of gaboxadol, described as a selective extrasynaptic GABAA agonist, were discontinued because of intolerable adverse effects. The development of indiplon, a BZRA hypnotic with a short half-life evaluated in immediate- and modified-release formulations, was discontinued after FDA requests for further studies.
Potential new BZRA hypnotics. The BZRA hypnotics now include those with the benzodiazepine structure, which are relatively nonselective for GABAA subunit subtypes, and the nonbenzodiazepine compounds, which have varying degrees of selectivity for α subtypes. All are immediate-release formulations, with the exception of zolpidem extended-release. As noted above, modifying the release of a hypnotic has been a key pharmacokinetic innovation among sleep medications. Although other BZRA modified- release formulations have been evaluated, none appear to be actively investigated at present.
The other major pharmacokinetic innovation in development is the creation of rapidly absorbed alternate-delivery formulations of BZRA hypnotics. In addition, BZRA compounds with different subtype selectivity are being investigated as possible insomnia treatments.
The argument for BZRA alternate-delivery formulations is they can use oral, nasal, or pulmonary routes to allow more rapid onset of action. An earlier maximum serum concentration of the drug would be accompanied by an earlier decline in the sedating effect, which may help avoid undesired residual sedation. This combination would make such a hypnotic ideal for middle-of-the-night (MOTN) dosing. Currently, none of the insomnia medications is approved for MOTN use and, therefore, cannot be marketed for this indication. Since sleep maintenance difficulty is the most common nighttime insomnia symptom, a medication taken after a nighttime awakening would seem attractive.
The alternate-delivery formulations now being investigated are all based on medications with relatively short half-lives now available in pill form. Most are zolpidem formulations; however, some are based on zaleplon and triazolam. Among the alternate-delivery systems that have been studied are sublingual zolpidem formulations, zolpidem oral dissolving tablet, zolpidem oral spray, inhalation zaleplon, and triazolam nasal spray. The potential benefits of these rapid-acting formulations will need to be balanced against possible specific risks associated with their rapid onset of action.
The available nonbenzodiazepine BZRA hypnotics all have some degree of preferential binding to GABAA receptor complexes containing the α1 subunit subtype, compared with benzodiazepines with similar affinity for multiple α subtypes. It has been suggested that this subtype selectivity improves the tolerability and safety of the nonbenzodiazepine hypnotics. Eszopiclone also exhibits preference for the α3 subtype. Both α1 and α3 subtypes are associated with sedation.
In contrast, compounds with α2 selectivity are anxiolytic but are not sedating. Accordingly, medications with greater α1 or α3 preference might represent advances within the general class of BZRA hypnotics. One unique compound being investigated for the treatment of insomnia is adipiplon, a GABAA α3 partial agonist.
All of the FDA-approved BZRA hypnotics have been characterized as pharmacodynamic antagonists. In addition to the investigational compound adipiplon, clinical trials recently have been performed with other partial agonists. One example is EVT 201, which is reported to be a partial positive allosteric modulator of the GABAA receptor complex.
Melatonin receptor agonists. Ramelteon is the only FDA-approved melatonin receptor agonist. Although various melatonin formulations are marketed as dietary supplements, these preparations are unregulated and have not been comprehensively evaluated for efficacy and safety in the treatment of insomnia. However, at least 4 other compounds with agonist activity at the melatonin receptors have been investigated as possible insomnia medications. Examples include BDD-40001, TIK-301, and VEC-162. Agomelatine is a melatonin receptor agonist with serotonin (5-HT)2C antagonist activity that is being developed as an antidepressant; however, it may have therapeutic effects for insomnia symptoms.
5-HT2A receptor antagonists and related compounds. Several medications with postsynaptic 5-HT receptor antagonist activity are associated with sedation, and some have been shown to increase the amount of slow wave sleep. It has been hypothesized that greater slow wave sleep will improve the quality of nighttime sleep as well as daytime alertness.
Although no medication in this class is FDA-approved for the treatment of insomnia, some antidepressants and antipsychotics prescribed for patients with insomnia incorporate 5-HT2 antagonism, especially with the 5-HT2A and 5-HT2C receptor subtypes. Examples include trazodone and mirtazapine, although these medications have multiple receptor effects that may contribute to undesired effects.
There has been considerable interest in the development of 5-HT2A receptor antagonists for the treatment of insomnia. An appropriately selective compound with a moderate duration of action that avoids the adverse effects of medications currently prescribed on an off-label basis might represent a good candidate. At least 9 different compounds with 5-HT2A antagonism and related receptor effects have been investigated as possible insomnia medications. Some compounds are being evaluated in phase 3 studies. Trials have been conducted with 5-HT2A receptor antagonists, 5-HT2A inverse agonists, a compound with combined 5-HT2A and histamine (H)1 antagonism, and a tetracyclic with 5-HT2A, H1, and a2 receptor effects.
Orexin antagonists. The excitatory neuropeptides termed “orexins” or “hypocretins” first were described 10 years ago. The neurons originating in the lateral hypothalamus have widespread projections to several brain regions. The discovery of the deficiency of orexins in the sleep disorder narcolepsy highlighted the stimulatory role that these compounds normally play in helping to stabilize wakefulness. It was hypothesized that if low orexin activity was associated with excessive sleepiness, then decreasing orexin functioning might promote sleep, at least for some patients who have insomnia.15
At least 2 compounds that have pharmacodynamic characteristics of orexin antagonists have been investigated as possible insomnia treatments. Phase 3 studies are currently evaluating the orexin antagonist almorexant. Preliminary studies have shown that almorexant is well tolerated and provides benefits in sleep onset, sleep maintenance, and sleep efficiency.16
Histamine H1 receptor antagonists. Histamine is a wake-promoting neurotransmitter. Histaminergic neurons originate in the hypothalamic tuberomammillary bodies and have widespread projections. Antihistamines are the active ingredients of OTC sleep aids; diphenhydramine is the most common OTC antihistamine, but doxylamine is in some of these products. Although marketed as sleep aids, these products have not been evaluated comprehensively for safety and efficacy in patients with chronic insomnia.
Disadvantages of the current OTC antihistamines are their relatively long duration of action and next-morning grogginess, potential tolerance to the sedating effects, and insufficient receptor selectivity. In addition to postsynaptic histamine receptor blockade, the OTC antihistamines are associated with muscarinic antagonism and the risk of undesired anticholinergic effects. Thus, more selective and centrally acting H1 receptor antagonists that have a shorter half-life might represent more desirable sleep-promoting agents. Theoretically, a compound functioning as an agonist at the presynaptic H3 autoreceptor might decrease histamine-related stimulation and therefore promote sleep.17
The antidepressant doxepin is known to have pronounced central antihistaminic activity. Although not approved specifically for the treatment of insomnia, doxepin has been prescribed at moderately low doses (25 to 50 mg) for its sleep-promoting effects. Phase 3 trials of doxepin at doses of 1, 3, and 6 mg have demonstrated efficacy in sleep onset and maintenance, as well as a positive safety and tolerability profile.18
α2 δ Calcium channel modulators. Medications in this category bind to voltage-sensitive calcium channels and may inhibit the release of several different neurotransmitters. The first medication in this category was gabapentin, which has indications for the treatment of epilepsy and postherpetic neuralgia.
Another example is pregabalin, which has indications for treating various pain syndromes and partial onset seizures but also has been evaluated for the treatment of insom- nia. A new compound, PD-200390, has also been studied as a possible insomnia medication. These compounds may be especially interesting because they may increase the amount of slow wave sleep.
Miscellaneous compounds. Several unique compounds have been evaluated in preclinical and clinical studies to determine their potential as pharmacotherapeutic therapies for insomnia. Among these is a neurokinin1 receptor antagonist (GW679769), a 5-HT1A agonist (MN-305), a 5-HT6 antagonist (RO-4368554), and E-6199 (with an unknown mechanism of action). Oth- er pharmacological categories that might represent fruitful research directions for insomnia treatment include corticotropin-releasing hormone antagonists, cytokines, glutamatergic antagonists, adenosine enhancers, and H3 agonists.
Summary
The past several years have witnessed significant advances in the pharmacological management of insomnia. Several new medications have become available, which represent pharmacokinetic and pharmacodynamic innovations, and a multitude of potential new insomnia treatments are being investigated. The challenge for the future will continue to be the optimization of efficacy and safety in the promotion of nighttime sleep and daytime functioning. Improvement in the quality of life for insomnia sufferers must be the ultimate goal.
References:
References
1. Neubauer DN. The evolution and development of insomnia pharmacotherapies. J Clin Sleep Med. 2007;3(5 suppl):S11-S15.
2. National Institutes of Health. National Institutes of Health State of the Science Conference statement on Manifestations and Management of Chronic Insomnia in Adults, June 13-15, 2005. Sleep. 2005;28:1049-1057.
3. Roth T, Walsh JK, Krystal A, et al. An evaluation of the efficacy and safety of eszopiclone over 12 months in patients with chronic primary insomnia. Sleep Med. 2005;6:487-495.
4. Krystal AD, Walsh JK, Laska E, et al. Sustained efficacy of eszopiclone over 6 months of nightly treatment: results of a randomized, double-blind, placebo-controlled study in adults with chronic insomnia. Sleep. 2003;26:793-799.
5. Krystal AD, Erman M, Zammit GK, et al; ZOLONG Study Group. Long-term efficacy and safety of zolpidem extended-release 12.5 mg, administered 3 to 7 nights per week for 24 weeks, in patients with chronic primary insomnia: a 6-month, randomized, double-blind, placebo-controlled, parallel-group, multicenter study. Sleep. 2008;31:79-90.
6. DeMicco M, Wang-Weigand S, Zhang J. Long-term therapeutic effects of ramelteon treatment in adults with chronic insomnia: a 1 year study. Sleep. 2006;29(suppl):A234. Abstract.
7. Zammit GK. The prevalence, morbidities, and treatments of insomnia. CNS Neurol Disord Drug Targets. 2007;6:3-16.
8. Möhler H, Fritschy JM, Rudolph U. A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002;300:2-8.
9. Greenblatt DJ. Pharmacokinetic determinants of hypnotic drug action: the art and science of controlling release. Sleep Med. 2006 May 12; [Epub ahead of print].
10. Moen MD, Plosker GL. Zolpidem extended- release. CNS Drugs. 2006;20:419-428.
11. Lankford A, Ancoli-Israel S. Indiplon: the development of a novel therapy for the treatment of sleep onset and sleep maintenance insomnia. Int J Clin Pract. 2007;61:1037-1045.
12. Kato K, Hirai K, Nishiyama K, et al. Neurochemical properties of ramelteon (TAK-375), a selective MT1/MT2 receptor agonist. Neuropharmacology. 2005;48:301-310.
13. Johnson MW, Suess PE, Griffiths RR. Ramelteon: a novel hypnotic lacking abuse liability and sedative adverse effects. Arch Gen Psychiatry. 2006;63:1149-1157.
14. McGechan A, Wellington K. Ramelteon. CNS Drugs. 2005;19:1057-1067.
15. Nishino S. The hypocretin/orexin receptor: therapeutic prospective in sleep disorders. Expert Opin Investig Drugs. 2007;16:1785-1797.
16. Brisbare-Roch C, Dingemanse J, Koberstein R, et al. Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat Med. 2007; 13:150-155.
17. Tashiro M, Yanai K. Molecular imaging of histamine receptors in the human brain [in Japanese]. Brain Nerve. 2007;59:221-231.
18. Roth T, Rogowski R, Hull S, et al. Efficacy and safety of doxepin 1 mg, 3 mg, and 6 mg in adults with primary insomnia. Sleep. 2007;30:1555-1561.
19. Walsh JK, Roehrs T, Roth T. Pharmacologic treatment of primary insomnia. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 4th ed. Philadelphia: Elsevier Inc; 2005: 749-760.
