- Clinical Technology
- Adult Immunization
- Hepatology
- Pediatric Immunization
- Screening
- Psychiatry
- Allergy
- Women's Health
- Cardiology
- Pediatrics
- Dermatology
- Endocrinology
- Pain Management
- Gastroenterology
- Infectious Disease
- Obesity Medicine
- Rheumatology
- Nephrology
- Neurology
- Pulmonology
How Do Medical and Pharmacy Directors Perceive the Value of New Cancer Drugs?
A total of 50 health care professionals, including 25 health plan medical directors, 20 health plan pharmacy directors, and 5 pharmacy directors for pharmacy benefit management companies were surveyed regarding their perceptions of the value of 3 novel cancer therapies. The physicians and pharmacists were asked to estimate the monthly average wholesale price of each therapy, overall survival benefit of bevacizumab for treatment of persons with advanced colorectal cancer and erlotinib for treatment of persons with non–small-cell lung cancer, and progression-free survival benefit of sunitinib malate for treating persons with advanced renal cell carcinoma. Most respondents overestimated drug costs and underestimated survival benefit associated with these treatments. Mean incremental cost-effectiveness ratio for all drugs studied was approximately $170,000/quality-adjusted life-year (QALY). Cost-effectiveness ratios were lower than the $300,000/QALY cited by oncologists in another study but significantly higher than those for many other costly interventions. Our study findings reflect the need for a better understanding of the value of the clinical benefits of novel cancer therapies in an environment of product innovation but with resource constraints. (Drug Benefit Trends. 2009;21:120-130)
Novel cancer therapies make up the greatest percentage of new drugs in development, with nearly 750 new cancer drugs and new indications for existing cancer drugs in various stages of clinical development.1 However, there is cost associated with innovation. The average cost of a cancer drug prescription rose nearly 16% in 2005 compared with 3% for other prescriptions, with the average cost of a 30-day prescription for a cancer medication totaling nearly $1600.2 Furthermore, cancer drugs account for an increasing share of total dollars spent on medications.3 While antineoplastic products constituted 16% of the total cost of US prescriptions in 2004, the National Cancer Institute projected that these agents would consume almost a quarter of overall drug spending by 2007.4 An analysis of an administrative pharmacy claims database found that spending on oral chemotherapy drugs as a proportion of total pharmacy benefit costs more than doubled between 2002 and 2006, from 0.3% to 0.7%.5
Although use of these novel therapies has increased overall survival rates, some may view the gains achieved to be modest relative to the overall cost of treatment.6 Improvements in overall survival are critical to the advancement of cancer treatment, but there has been criticism that these survival gains are marginal.7
How payers, patients, and health care providers assess the value of new therapies in light of rising costs during the next decade has significant implications for advancing the treatment of persons with cancer. A survey by Nadler and colleagues8 of 139 medical oncologists at 2 large academic hospitals found that 78% said patients should have access to effective care regardless of cost. However, 71% of respondents said that rising costs would result in more rationing in the area of oncology treatment during the next 5 years.8
Using a specific cancer drug scenario, the average implied cost-effectiveness threshold for the oncologists surveyed by Nadler was $300,000 per quality-adjusted life-years (QALYs) gained, significant- ly higher than the threshold of $50,000/QALY to $100,000/QALY that has been cited in studies of other costly interventions, including bone marrow transplant, cardiac transplant, and automated implantable cardioverter defibrillator.9-12
The objective of this study was to determine third-party payers’ perceptions of the value associated with 3 cancer therapies: bevacizumab (Avastin), erlotinib (Tarceva), and sunitinib malate (Sutent) via survey research conducted among medical and pharmacy directors of health plans and pharmacy benefit management (PBM) companies, and to compare these results with the perceptions of oncologists reported by Nadler and colleagues.
Materials and Methods Participants. From November 1 through December 31, 2006, we recruited and interviewed by telephone 25 medical directors and 20 pharmacy directors from a nationally representative sample of managed care organizations. In addition, we interviewed 5 pharmacy directors from PBMs. The interviews lasted 1 hour. An incentive of $200 was offered to the participants who were assured of confidentiality.
Questionnaire. The questionnaire consisted of 5 sections designed to evaluate the perception of the value of novel cancer therapies from a third-party-payer perspective. In section 1, respondents were asked to provide demographic information and general health plan formulary information.
Section 2 assessed perceptions of the value of novel therapies by asking respondents to imagine a hypothetical, newly approved medication (Product X) for treatment of patients with metastatic non–small-cell lung cancer (NSCLC). In one scenario, respondents were told to assume that the new treatment costs $8000 per month ($96,000 per year, or $66,000 more than the standard of care for 1 year). Respondents were then asked to identify the minimum overall survival benefit offered by the new medication at which they would be prepared to include the medication on the plan’s formulary in addition to standard-of-care treatment. In a second scenario, the same question was posed for a new treatment costing $5000 per month ($60,000 per year, or $30,000 per year more than the standard of care). In both scenarios, respondents were told to assume no differences in quality of life or adverse-effect profile between the treatments.
In section 3, without referring to published information, respondents were asked to estimate the mean overall survival benefit and cost per month associated with the use of bevacizumab for treatment of persons with advanced colorectal cancer (CRC) and erlotinib for treatment of persons with NSCLC, as well as mean progression-free survival (PFS) benefit and per month costs associated with the use of sunitinib malate for treatment of persons with advanced renal cell carcinoma (RCC). (PFS data were used for sunitinib because overall survival data were not available at the time this study was conducted.) A mean overall survival benefit is the average overall increase in patient life span, whereas a mean PFS benefit is the average increase in length of time that the patient’s condition does not worsen.
Respondents were then asked whether they believed each drug offered “good value for the money.” Responses to this follow-up question were captured on a modified 5-point Likert scale (with responses ranging from “yes, definitely” to “definitely not”). After applying the approach used in the Nadler study, implied cost-effectiveness ratios were calculated using the metric of dollars per QALY ($/QALY). Respondents were then presented with actual cost and survival information from the product label and were again asked whether they thought the drug provided good value.
Section 4 evaluated respondents’ impressions of the incremental cost-effectiveness of oral cancer agents as adjuvant treatments. Respondents were asked whether a hypothetical new oral drug used in the adjuvant setting that provided an absolute increase in 5-year survival of 5% above the standard of care would be covered if the drug cost $5000 per month more than the standard of care, or if it cost $10,000 per month more than the standard of care. They were asked how much the drug would be worth in terms of cost per month, assuming that 1 year of use as an adjuvant treatment would be necessary. Respondents were then asked whether the new drug would offer good value if it cost $8350 per month more than the standard of care for the same 5% benefit in 5-year survival.
In section 5, we explored the effect of patients’ prescription drug copays. Respondents were asked whether the overall cost of new cancer medications or out-of-pocket costs faced by their patients influenced their formulary recommendations. They were also asked whether they believed every patient should have access to effective cancer treatments regardless of cost. Each response in this section was also recorded on a 5-point Likert scale (responses ranging from strongly agree to strongly disagree).
Data analysis. Responses in section 2 were used to calculate incremental cost-effectiveness ratios (ICERs). Responses in section 3 were used to calculate implied cost-effectiveness ratio (CER) using the metric of $/QALY as defined by Nadler and colleagues.8
In section 2, which assessed perceived cost-effectiveness of hypothetical treatments, ICERs were calculated for 2 scenarios using the following formula: ICER = Δ Cost/ Δ QALY, where:
• Δ Cost = Annual cost above the standard of care (scenario A = $66,000; scenario B = $30,000).
• Life expectancy (survival) is indicated in life-years (LY); if ranges were provided, the midpoint was used. For example, if a respondent described additional survival time as 1 to 3 months, a midpoint of 2 months was used.
• Quality adjustment (QA) = 1 (ie, no change in quality of life).
• A sample calculation (assuming the interviewee selected an additional survival time of 6 months as follows for scenario A) was as follows: ICER = $66,000/(6/12) 3 1 = $132,000/QALY).
• In our determination of ICERs, we assumed the standard of care would be 1 full year.
In section 3, implied CERs were calculated using the following formula: CER = Annual cost/D QALY, based on the respondents’ estimates of cost and survival benefits associated with the newer treatments.
Results Section 1. The 50 respondents were all experienced professionals who had been in their current position for an average of 8.1 years. Nine respondents represented national plans or PBMs with an average of 20.7 million covered lives. The remaining 41 represented regional health plans or PBMs with an average of 1.03 million covered lives. Sixty-eight percent reported that their plans used multiple benefit designs. Eighty-six percent (43/50) used a 3-tiered benefit plan design (copays ranged from $10 to $20 for medications on tier 1, $20 to $35 for tier 2, and $30 to $55 for tier 3); 50% (25/50) used a 2-tiered benefit plan design (copays ranged from $0 [Medicaid] to $15 for tier 1 and from $5 [Medicaid] to $35 for tier 2); and 30% had a 4-tiered benefit plan design, with tier 4 medications, including biologics/specialty pharmacy injectables (copays ranging from $7 to $100 or 20% to 50% of drug cost).
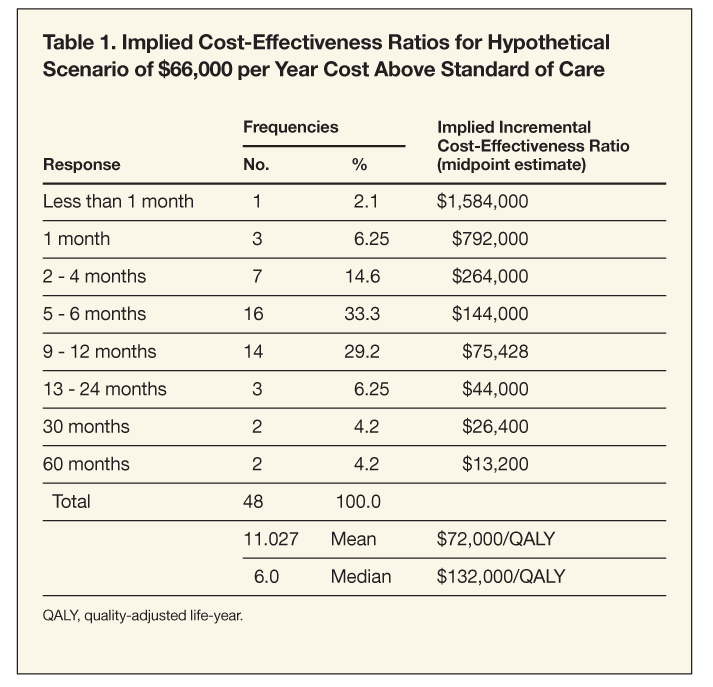
Section 2. Respondents reported that Product X would represent a good value at $66,000 more per year only if a minimum improvement of 10.8 months in overall survival above standard of care was expected. They indicated that an improvement of 9 months in overall survival would justify an additional cost of $30,000 more per year. Only 22% of respondents expected a minimum improvement in survival up to 4 months for a drug that cost $66,000 per year above standard-of-care treatment. These responses translated into a mean (median) ICER of $72,000/QALY ($132,000) for the $66,000 more per year scenario and $40,000/QALY ($60,000) for the $30,000 more per year scenario. ICERs are presented in Table 1.
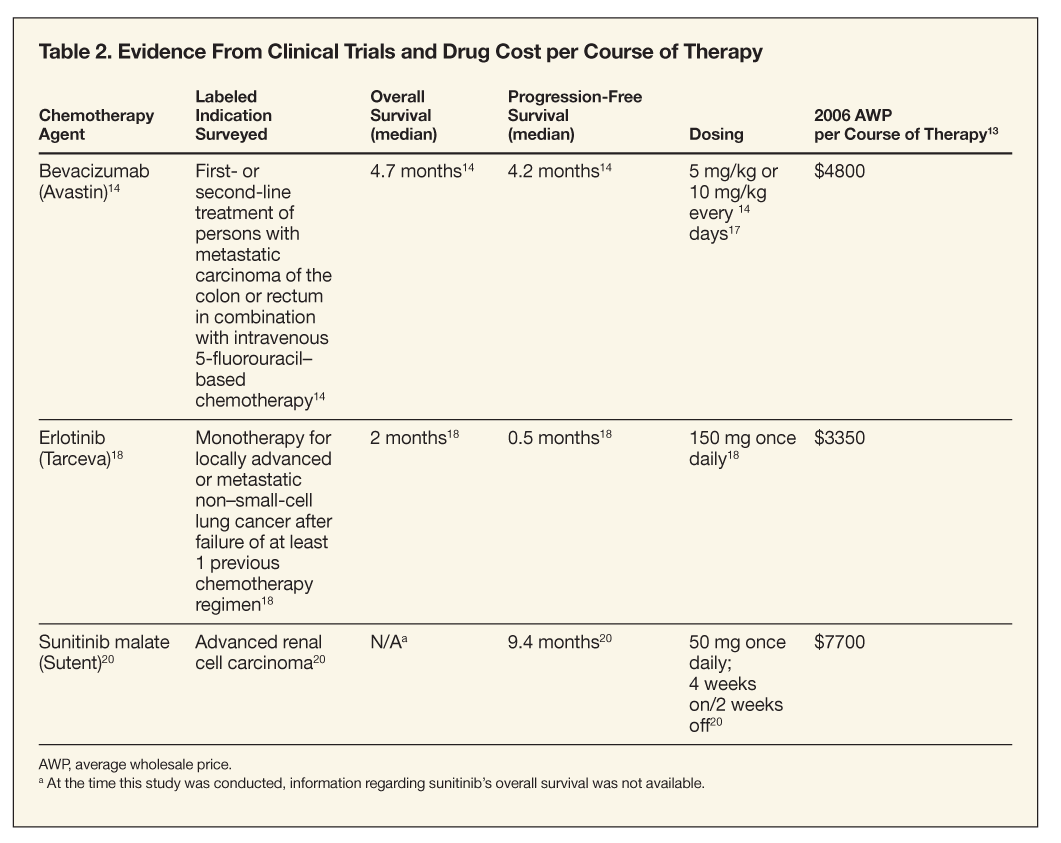
Section 3.Cost-effectiveness of bevacizumab, erlotinib, and sunitinib. At the time of this research, average wholesale prices (AWPs) for bevacizumab, erlotinib, and sunitinib per month were approximately $4800, $3350, and $7700, respectively (Table 2).13 Clinical efficacy of bevacizumab and erlotinib has been demonstrated, with a mean increase in median overall survival ranging from 2 to 4.7 months and a mean increase in PFS benefit ranging from 0.4 to 4.4 months for most solid tissue malignancies, including cancers of the colon, breast, and lung.14-20 At the time of this study, overall median survival time for sunitinib was unknown, and the median PFS benefit associated with sunitinib for the treatment of persons with advanced RRC was approximately 9.4 months (range, 27.1 to 54 weeks).20
On the basis of previous research, we deemed the median overall survival benefit used to calculate ICERs for bevacizumab to be approximately 4.7 months longer than standard care in patients with CRC,14,15 and the median overall survival benefit for erlotinib to be approximately 2 months longer than best supportive care for patients with refractory NSCLC.18,19 The median PFS benefit used to calculate ICERs for sunitinib for the treatment of persons with advanced RCC was approximately 9.4 months.20
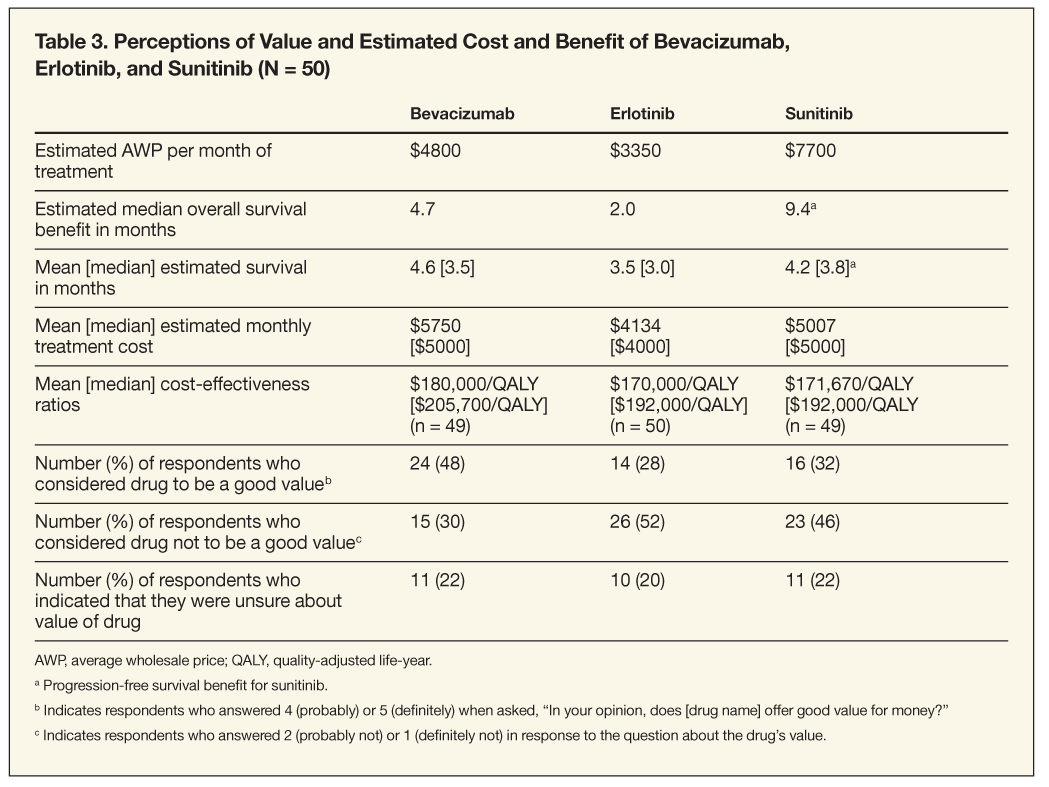
For bevacizumab, slightly more than half (51%) of respondents underestimated survival benefit, but the mean response was only 0.1 month less than the actual overall median increase in survival for bevacizumab (Table 3). The cost of bevacizumab therapy was overestimated by 50% of respondents, but only slightly: the average response of $5750 exceeded the actual average cost for 1 month of bevacizumab therapy by about 20%.
Many respondents reported a lack of familiarity with sunitinib but still attempted to answer the questions pertaining to this novel therapy. All respondents underestimated sunitinib’s midpoint median PFS benefit of 9.4 months, but 22.5% gave an estimate close to the actual PFS-ie, 6 months or more. The vast majority of respondents (94%) underestimated sunitinib’s monthly cost; the average response was 35% lower than the actual average monthly cost of sunitinib.
By contrast, two-thirds of respondents overestimated the survival benefit for erlotinib by 2 months (mean response was 3.5 months of additional benefit), and 58% overestimated its monthly cost. With regard to monthly cost, the average response was $4134, which is 23% above the actual average cost for 1 month of erlotinib therapy.
Taking into account all respondents’ answers, the implied CER for bevacizumab was approximately $180,000/QALY based on a mean estimated survival time of 4.6 months (Standard Deviation [SD] = 2.9) and a mean estimated monthly cost of $5750. For erlotinib, the mean CER estimate was about $170,000/QALY based on a mean estimated survival time of 3.5 months (SD = 1.8) with a mean estimated monthly treatment cost of $4134. The mean CER estimate for sunitinib was approximately $171,670/QALY based on a mean estimated PFS of 4.2 months (SD = 2) and a mean estimated monthly treatment cost of $5007 (Table 3).
Perceived value of bevacizumab, erlotinib, and sunitinib. After being informed of the actual monthly cost and median survival increase for bevacizumab, 48% of respondents believed the cost of 1 month’s treatment with bevacizumab definitely or probably offered good value for the money, while 30% felt it did not offer good value; the rest were unsure (Table 3). A greater percentage of those who deemed bevacizumab not to be a good value had initially underestimated its survival benefit and overestimated its cost compared with respondents who considered it to be a good value. For those who did not consider bevacizumab to be a good value, the mean additional survival time they felt would be required to make it a good value was 11.2 months beyond the actual 4.6 months.
Twenty-eight percent of respondents believed that treatment with erlotinib for NSCLC definitely or probably offered good value for the money, versus 52% who felt it probably or definitely did not. The latter group reported they would require a mean additional survival time of 6.7 months to consider erlotinib as being of good value. Twenty percent of respondents were unsure about whether erlotinib offered good value. Respondents who did not deem erlotinib to represent a good value tended initially to overestimate its survival benefit and overestimate its monthly cost versus those who considered the drug to be a good value.
Only 32% of respondents deemed sunitinib as definitely/ probably offering good value on the basis of its cost for 1 month’s treatment for RCC, whereas 46% said it was probably/definitely not a good value; 22% were unsure. Some respondents cited sunitinib as offering good value because of its high PFS benefit and the lack of any alternatives. Those who did not consider sunitinib to be a good value tended initially to underestimate its PFS benefit and underestimate its monthly cost versus those who did consider it to be a good value or were unsure. Those who were unsure or who believed sunitinib did not provide good value would require an average of 12.6 months beyond its actual 9.4 months of PFS.
Section 4: Use in adjuvant setting. When asked to evaluate a hypothetical cancer therapy (Product Y) for metastatic NSCLC in an adjuvant setting that would increase 5-year survival rate by 5%, 54% of respondents (27/50) indicated they definitely/probably would cover this product, whereas 28% said they probably/definitely would not; 18% of respondents were unsure. When a cost of $5000 or $10,000 per month above the standard of care (with total treatment duration of 1 year) was linked to the product, the percentage of respondents willing to reimburse for the product decreased to 38% and 12%, respectively. Respondents were then told to assume Product Y had been shown in a large randomized trial to benefit patients with NSCLC in the adjuvant setting with a 5% absolute increase in 5-year survival above the standard of care. They were told to assume the cost of this product to be $8350 per month, $100,000 more per year than the standard of care. Only 6% of respondents believed this product definitely or probably offered good value. The majority of respondents considered this product too costly under the assumption that it would confer only a 5% benefit in overall survival. The average cost per month that respondents were willing to pay for Product Y in the adjuvant setting was $2946.
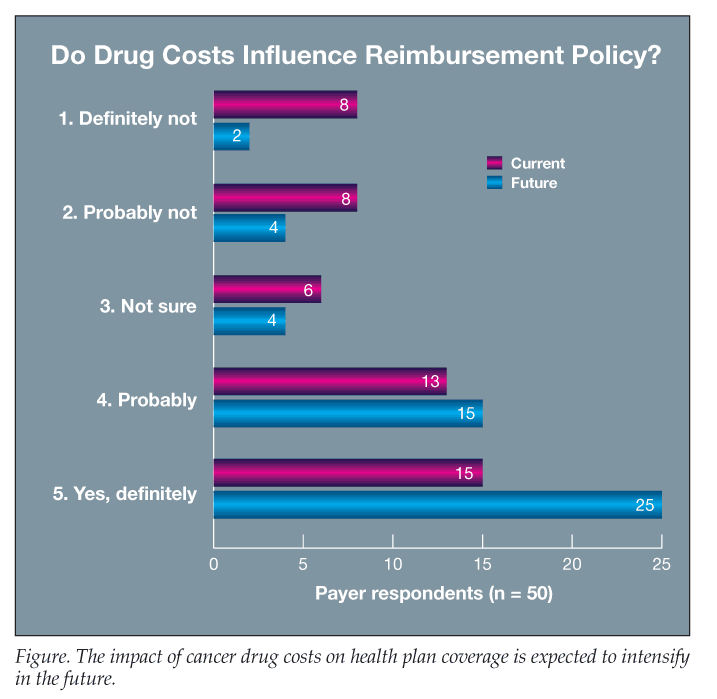
Section 5: Role of treatment costs. Fifty-six percent of respondents indicated that probably or definitely that current drug costs influence their decisions regarding which cancer treatments would receive optimal reimbursement with minimal access restrictions, compared with 32% who indicated probably not or definitely not; 12% were unsure (Figure). Eighty percent of respondents agreed that, in the future, costs will play a more important role in their plan’s decisions about which cancer treatments to cover without restrictions compared with just 12% who disagreed and 8% who were unsure.
Sixty-four percent of respondents probably or definitely agreed that every patient should have access to effective cancer treatments, regardless of cost, versus 22% who disagreed; 12% were unsure. However, many respondents commented that providing “access” to a treatment did not necessarily imply full plan coverage.
Eighty-two percent (41/50) indicated that they currently have or plan to implement restrictions on the coverage/reimbursement of FDA-approved oncology medications. The most commonly cited restrictions anticipated were increased use of coinsurance, prior authorizations using guidelines or step therapy, treatment monitoring, and noncoverage of off-label use of certain FDA-approved oncology products.
Discussion
The high cost of new cancer thera-pies is beginning to influence third-party-payers’ decisions on coverage and reimbursement trends. Recent research has focused on the impact of the cost of oral cancer therapies on pharmacy and health plan budgets. An analysis of an administrative pharmacy claims database by Curtiss5 found that spending on oral chemotherapy drugs as a proportion of total pharmacy benefit costs more than doubled between 2002 and 2006, from about 0.3% to 0.7%. Ramsey and colleagues21 developed a budget impact model to estimate the impact on health plan budgets during 1 year after introduction of erlotinib as second- and third-line therapy for patients with advanced NSCLC; they concluded that inclusion of erlotinib on a formulary has a relatively small impact on the annual health care budget or per member per month (PMPM) expenditures if used according to product label indications. The model did not, however, take into account the use of erlotinib for other indications or off-label use.21
In the present study, 56% of respondents indicated that drug costs currently influence their decisions regarding which cancer treatments would receive optimal reimbursement with minimal access restrictions, but a much higher percentage (80%) of respondents indicated that costs will play a more important role in their plan’s decisions regarding which cancer treatments to cover without restrictions in the future.
When health plan/PBM directors were asked to cite the minimum improvement in median overall survival that they felt would be necessary to confer good value to a hypothetical new cancer medication, the median response was 6 months- regardless of whether Product X was priced at $5000 per month or $8000 per month above the standard of care. When asked about specific rather than hypothetical medications, in most cases, respondents indicated that their expectations for survival benefit were much higher than the actual survival benefit of the novel cancer agents evaluated. This suggests a cost insensitivity and indicates that PBMs are more sensitive to outcome or intervention benefit than cost.
Studies of the clinical efficacy of novel cancer agents have reported median overall survival benefit ranging from 2 months with erloti- nib19 to 9.4 months PFS benefit with sunitinib malate for the treatment of persons with advanced RCC.20 Respondents who did not view the drugs in our study to represent a good value required additional survival benefits that were out of proportion to any potential survival advances that could be expected in the near future. For example, respondents who did not view bevacizumab to represent a good value required an additional survival benefit of 12 months for patients with NSCLC or a doubling of the most recent data. Most of our payer respondents overestimated the survival benefit associated with the new therapies, suggesting they were not as concerned about cost as they were in seeing increases in survival time and PFS associated with novel cancer treatments.
In the Nadler study, oncologists most likely had more experience with the novel agents and therefore had a more realistic expectation of survival benefits seen with newer agents and were willing to prescribe these drugs despite the costs incurred to derive these benefits. In that study, 62% believed that a life-expectancy gain of at least 2 to 4 months justified the use of a hypothetical novel agent that cost $70,000 per year above the standard of care; an additional 20% felt that 4 to 6 months would justify this cost. The mean implied CER was $280,000. Only 1 respondent replied that 1 day of additional life would justify the $70,000 per year cost, implying a cost-effectiveness threshold of $25 million per QALY ($70,000 multiplied by 365).

Our payer respondents had a 53% lower mean implied cost-effectiveness threshold for the hypothetical Product X than did the on- cologists surveyed by Nadler. In addition, the mean implied cost-effectiveness threshold cited for bevacizumab was 44% lower for our our payers than for the oncolo- gists ($180,000/QALY vs $320,000/ QALY) (Table 4)-but higher than the $50,000/QALY standard often cited in assessments performed by health care technology assessment organizations in the European Union, Canada, and elsewhere.
The percentages of our respondents rating 3 novel cancer agents as offering a good value were 48% for bevacizumab, 28% for erlotinib, and 32% for sunitinib. These low responses are surprising because these 3 agents are considered to be breakthrough treatments.
In addition, perceived value of an agent did not always correspond to the survival benefit and/or cost of the agent. For example, a greater percentage of respondents considered bevacizumab to have a greater value than sunitinib even though sunitinib had more than twice the PFS benefit but only about a 37% higher cost than bevacizumab. This could be a result of greater familiarity with bevacizumab. Another explanation for payers not finding as much value for sunitinib is that the metric used to evaluate sunitinib was PFS, a less familiar metric than overall survival.
The payers in our study were less likely to agree that every patient should have access to effective cancer care, regardless of cost, compared with the oncologists surveyed by Nadler (64% vs 78%). Although a majority of the oncologists did not feel that treatment costs would influence their clinical decisions, 81% stated that they would consider a patient’s out-of-pocket costs in their ultimate recommendations. When asked if costs would play a more important role in the next 5 years, two-thirds of the oncologists concurred. Furthermore, 71% believed that during the next 5 years, rising costs could result in greater rationing.
Payers are more inclined than oncologists to believe the costs of new cancer drugs currently influence their decisions regarding which cancer treatments will receive optimal reimbursement (56% of the payers in our study vs 30% of the oncologists surveyed by Nadler). Similarly, a higher percentage of our respondents agreed that future costs of new cancer drugs would impose a greater need for coverage and utilization restrictions (88% vs 72% of oncologists).
Surprisingly, managed care directors did not value novel cancer agents when used in the adjuvant setting as they did when used as primary treatment of metastatic disease. The average cost per month of Product Y in the adjuvant setting that respondents were willing to pay was $2946-far lower than the cost for the 3 novel therapies discussed in the survey. One might postulate that the benefit of “curing” 5% more persons who had a given malignancy by improving 5-year survival rate would be valued at a level higher than extending a patient’s life by a few months.
The majority of respondents believed that a 25% patient copayment would enhance their willingness to offer novel cancer therapies. In the case of bevacizumab, 80% of respondents believed that bevacizumab should definitely or probably be offered as a treatment option. Similar findings were seen with erlotinib and sunitinib (78% and 72%, respectively). However, the 25% copayment did not meaningfully change respondents’ perception that novel drugs did not offer good value for the plan.
It is critical for third-party payers to have access to reliable quality and value measurements to help them determine whether new therapies improve patient outcomes with an acceptable cost-benefit ratio.22,23 As stated by Lipsy and colleagues, “pharmacy departments or response managers must not approach the cost of therapies for cancer in a vacuum, and should develop a context within which cost is one element of a larger picture.”23
Study Limitations
Our study had a number of limitations. First, this was a qualitative study and not a statistical analysis. Second, formulary decision making involves the participation of many experts, including in this case, oncologists. The responses would probably have been different and more closely aligned to “real-life” situations if the respondents had been given the drug information in advance of the interview. Third, as with any survey, the manner in which the questions are framed may influence the responses.18 Bias was minimized by limiting the number of interviewers and using a scripted questionnaire. A fourth limitation was the method in which we calculated QALYs-ie, we evaluated cost and survival in terms of CER; however, we assumed there was no impact of quality of life and therefore the quality adjustment was held constant throughout all calculations. We believe these limitations had a minimal impact on our overall conclusions.
Conclusion
Commercial payers have only recently been exposed to the high cost of new cancer drugs, driven during the past 3 years by the introduction of several novel oral agents, concurrent with the introduction of commercially managed Medicare prescription drug plans. Our study findings reflect the need for a better understanding of the value of the clinical benefits of novel cancer therapies in an environment of increasing innovation with resource constraints. Data on the value of new therapies can help payers make better-informed decisions when drafting and implementing their coverage and reimbursement policies.
Acknowledgments The authors would like to thank Victoria Porter of i3 Innovus for her assistance in the preparation of this article.
This study was supported by AstraZeneca.
References:
References
1. Pharmaceutical Research and Manufacturers of America: Medicines in Development for Cancer. 2008. http://www.phrma.org/files/meds_in_ dev/Cancer2008.pdf. Accessed March 19, 2009.
2. Szabo L. Prices soar for cancer drugs. USA Today. July 11, 2006. http://www.usatoday.com/ news/health/2006-07-10-cancer-costs_x.htm. Accessed March 6, 2009.
3. Bach PB. Limits on Medicare’s ability to control rising spending on cancer drugs. N Engl J Med. 2009;360:626-633.
4. Minkoff NB. Colorectal cancer: complexities and challenges in managed care. J Manag Care Pharm. 2007;13(6, suppl C):S27-S29. http://www.amcp. org/data/jmcp/Pages%2027-29.pdf. Accessed March 6, 2009.
5. Curtiss FR. Pharmacy benefit spending on oral chemotherapy drugs. J Manag Care Pharm. 2006; 12:570-577. http://www.amcp.org/data/jmcp/ contemporary_subjects_570_577.pdf. Accessed March
6, 2009. 6. Johnson JR, Cohen M, Sridhara R, et al. Approval summary for erlotinib for treatment of patients with locally advanced or metastatic non-small cell lung cancer after failure of at least one prior chemotherapy regimen. Clin Cancer Res. 2005; 11: 6414-6421.
7. Ramsey SD. How should we pay the piper when he’s calling the tune? On the long-term affordability of cancer care in the United States. J Clin Oncol. 2007;25:175-179.
8. Nadler E, Eckert B, Neumann PJ. Do oncologists believe new cancer drugs offer good value? Oncologist. 2006;11:90-95.
9. Lee SJ, Anasetti C, Kuntz KM, et al. The costs and cost-effectiveness of unrelated donor bone marrow transplantation for chronic phase chronic myelogenous leukemia. Blood.1998;92:4047-4052.
10. Feldman AM, de Lissovoy G, Bristow MR, et al. Cost effectiveness of cardiac resynchronization therapy in the Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) trial. J Am Coll Cardiol. 2005;46: 2311-2321.
11. Chapman RH, Berger M, Weinstein MC, et al. When does quality-adjusting life-years matter in cost-effectiveness analysis? Health Econ. 2004;13: 429-436.
12. Zhang P, Engelgau MM, Norris SL, et al. Application of economic analysis to diabetes and diabetes care. Ann Intern Med. 2004;140:972-977.
13. Fleming T, ed. 2006 Red Book: Pharmacy’s Fundamental Reference (Red Book Drug Topics). Montvale, NJ: Thomson PDR; 2006.
14. Avastin (bevacizumab) prescribing information. Genentech BioOncology. http://www.avastin. com. Accessed March 6, 2009.
15. Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342.
16. Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer [published correction appears in N Engl J Med. 2007;356:318]. N Engl J Med. 2006;355:2542-2550.
17. Kabbinavar FF, Hambleton J, Mass RD, et al. Combined analysis of efficacy: the addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancer. J Clin Oncol. 2005;23:3706-3712.
18. Tarceva (erlotinib) prescribing information. Genentech USA, Inc and OSI Pharmaceuticals, Inc. http://www.tarceva.com. Accessed March 6, 2009.
19. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al; National Cancer Institute of Canada Clinical Trials Group. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353: 123-132.
20. Sutent (sunitinib malate) prescribing information. Pfizer Oncology. http://www.sutent.com. Accessed March 6, 2009.
21. Ramsey SD, Clarke L, Kamath TV, Lubeck D. Evaluation of erlotinib in advanced non-small cell lung cancer: impact on the budget of a US health insurance plan. J Manag Care Pharm. 2006; 12:472-478. http://www.amcp. org/data/jmcp/ form_472-478.pdf. Accessed March 6, 2009. 22. Establishing a single-source approach to measuring quality. Jt Comm Perspect. 2001;21(8):5.
23. Lipsy RJ, Fuller MG, Roski J, Mansukani S. Anticipating the future: how the emergence of innovative biologic agents impacts benefit design, utilization, and provider relations. J Manag Care Pharm. 2004;10(3 suppl):S4-S9. http://www. amcp.org/data/jmcp/MAY04% 20Supplement1.pdf. Accessed March 6, 2009.
