- Clinical Technology
- Adult Immunization
- Hepatology
- Pediatric Immunization
- Screening
- Psychiatry
- Allergy
- Women's Health
- Cardiology
- Pediatrics
- Dermatology
- Endocrinology
- Pain Management
- Gastroenterology
- Infectious Disease
- Obesity Medicine
- Rheumatology
- Nephrology
- Neurology
- Pulmonology
A Case of "Refractory Asthma"
A 43-year-old African American woman presented to the emergency department with severe dyspnea, wheeze, and cough productive of white sputum. Three years earlier, she had been given a diagnosis of asthma based on symptoms of wheeze and cough; her treatment regimen included intermittent use of albuterol.
A 43-year-old African American woman presented to the emergency department with severe dyspnea, wheeze, and cough productive of white sputum. Three years earlier, she had been given a diagnosis of asthma based on symptoms of wheeze and cough; her treatment regimen included intermittent use of albuterol.
The patient’s present symptoms began approximately 2 weeks ago and progressively worsened over the preceding 4 days. She denied chest pain, fever, and chills but noted extreme fatigue when attempting her daily activities. In addition to asthma, she has a 30-pack-year smoking history. Two primary care visits in the preceding 2 weeks resulted in empiric treatment for an infectious asthma flare with oral prednisone (30 mg once daily), azithromycin (7-day course), inhaled fluticasone, and as-needed albuterol inhaler. Her albuterol use had escalated to at least 6 times a day, with only modest symptom relief.
On initial examination, the patient was in extremis, sitting upright in bed, using accessory respiratory muscles, and unable to complete sentences because of severe dyspnea. Vital signs were as follows: temperature, 37°C (98.6°F); respiratory rate, 28 breaths per minute; heart rate, 120 beats per minute; and oxygen saturation on room air, 85%. Chest auscultation revealed bilateral diffuse coarse crackles and wheeze. A chest radiograph demonstrated prominence of the interstitial markings (Figure 1).
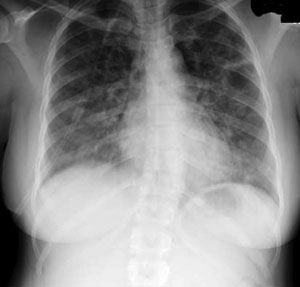
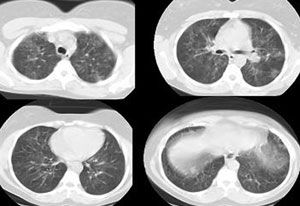
The patient was admitted with a tentative diagnosis of infectious exacerbation of asthma and pneumonia. She was treated with oxygen via nasal cannula (4 L/min); intravenous hydrocortisone, levofloxacin, and metronidazole; nebulized albuterol every 4 hours; and smoking cessation counseling. A pulmonary consultation was requested on day 5 because the patient did not have an appreciable clinical response to therapy. A CT scan of the chest revealed bilateral, diffuse, ground-glass opacities without significant air trapping (Figure 2).
Bronchoscopy was subsequently performed. Findings included clear secretions and thick mucous plugs. Bronchoalveolar lavage (BAL) and transbronchial biopsy specimens were obtained from the right upper and lower lobes. The BAL fluid was negative for fungi, Mycobacterium tuberculosis, and viruses; cytological examination of the specimens yielded negative results. Bacterial culture revealed rare Klebsiella pneumoniae that was sensitive to levofloxacin. The presumed diagnoses at this time remained asthma flare with diffuse mucous plugging and pneumonia caused by K pneumoniae.
The patient’s symptoms, exercise tolerance, and oxygen requirement improved gradually, and she was discharged 5 days later with portable oxygen, a 10-day oral prednisone taper, inhaled fluticasone, and nebulized albuterol. Outpatient pulmonary and smoking cessation follow-up appointments 1 week later were missed, and the patient did not return until 8 weeks later, when she reported that her respiratory status remained unchanged. Pulmonary function test results are shown in the Table.
Table – The patient’s pulmonary function test results
Spirometry
FEV1
1.48 L (69% predicted)
FVC
1.56 L (62% predicted)
FEV1:FVC
94% (111% predicted)
MVV
68 L/min (83% predicted)
Lung volumes
ERV
0.29 L (33% predicted)
FRC
2.15 L (59% predicted)
RV
1.27 L (65% predicted)
TLC
3.92 L (69% predicted)
Diffusion capacity
Dlco
8 mL/min/mm Hg (56% predicted)
FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; MVV, maximum voluntary ventilation; ERV, expiratory reserve volume; FRC, functional residual capacity; RV, residual volume; TLC, total lung capacity; Dlco, carbon monoxide–diffusing capacity.
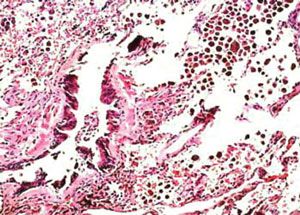
A repeated CT scan revealed persistent ground-glass infiltrates. The patient underwent a thoracoscopic biopsy. A low-power view of the specimen is shown in Figure 3.
What is causing this young woman’s asthma exacerbation and ground-glass infiltrates? How would you proceed?
ANSWER: Respiratory Bronchiolitis Interstitial Lung Disease (RB-ILD)
The low-power view of the biopsy specimen shows a terminal and a respiratory bronchiole surrounded by scar tissue and alveolar septal thickening. The presence of brown pigmented macrophages abundant in the airspace adjacent to the affected bronchioles led to a diagnosis of respiratory bronchiolitis interstitial lung disease (RB-ILD).
Discussion
RB-ILD was first described by Myers and colleagues1 in 1987 in their report on 6 heavy smokers who had clinical, radiological, and physical evidence of interstitial lung disease. Open lung biopsy specimens showed respiratory bronchioles with inflammatory cellular infiltration, thickened alveolar septa, and a clustering of light brown–pigmented macrophages in the airspaces.
A rare interstitial lung disease, RB-ILD is almost always associated with a history of smoking, with rare exceptions related to inhalation of noxious substances.2 RB-ILD may represent a progression of smoking-related respiratory bronchiolitis (smokers’ bronchiolitis), a very common asymptomatic histopathological finding, first described in 1974 by Niewoehner and associates.3
The distinction between smokers’ bronchiolitis and RB-ILD lies in the onset of symptoms and biopsy findings of interstitial inflammation and/or fibrosis. Both conditions share the characteristic findings of bronchiolar inflammation and a clustering of light brown–pigmented macrophages. Until 1989, the largest series of RB-ILD were from 2 studies involving a total of 24 patients.1,4 Since then, a handful of published case series have described 200 cases. Based on this collective experience, patients present in the fourth or fifth decade with dyspnea and cough that is sometimes productive, accompanied by mild restriction and reduction in carbon monoxide–diffusing capacity.
The chest radiographic findings are abnormal in 70% to 80% of cases.5 CT scans and chest radiographs most frequently demonstrate ground-glass opacities that are known to correlate with macrophage accumulation in the alveolar spaces and alveolar ducts.6
RB-ILD is similar in many respects to another interstitial lung disease, desquamative interstitial pneumonia (DIP). Many authors believe both diseases should be addressed as one under the term “smoking-related interstitial lung disease.” Both share a common presentation and radiological and pathological findings. Smoking is highly associated with both conditions, occurring in more than 90% of cases.6
The pathological distinction between RB-ILD and DIP pertains to the distribution and extent of involvement: the disease process in RB-ILD centers on the small airways with some extension into the airspace, in contrast to a more uniform and widespread pattern with DIP.7 This apparently simple differentiation is often difficult to make because of overlap.
Treatment of both RB-ILD and DIP includes smoking cessation, supportive care, and a consideration of corticosteroids. Case reports suggest remission of the disease process with complete smoking cessation,6,8 including avoidance of secondary tobacco smoke exposure. Many report remission with administration of corticosteroids over several months, but concluding efficacy is difficult because of the concurrent abstinence of smoking. The course of RB-ILD appears stable and benign, although radiological findings can persist for many years. Deaths have been reported in patients with DIP but not in those with RB-ILD.
Outcome in this case
The patient’s symptoms, exercise tolerance, and pulmonary function gradually improved following a regimen of oral prednisone for 14 months that was tapered down to 7.5 mg/d. Unfortunately, complete abstinence from tobacco has been difficult to achieve despite strong encouragement and education. We are optimistic that she will return to her former job in the near future. This case emphasizes the need to consider alternative diagnoses in a patient unresponsive to conventional asthma medications.9
References:
1. Myers JL, Veal CF Jr, Shin MS, Katzenstein AL. Respiratory bronchiolitis causing interstitial lung disease. A clinicopathologic study of six cases. Am Rev Respir Dis. 1987;135:880-884.
2. Moon J, du Bois RM, Colby TV, et al. Clinical significance of respiratory bronchiolitis on open lung biopsy and its relationship to smoking related interstitial lung disease. Thorax. 1999;54:1009-1014.
3. Niewoehner DE, Kleinerman J, Rice DB. Pathologic changes in the peripheral airways of young cigarette smokers. N Engl J Med. 1974;291:755-758.
4. Yousem SA, Colby TV, Gaensler EA. Respiratory bronchiolitis-associated interstitial lung disease and its relationship to desquamative interstitial pneumonia. Mayo Clin Proc. 1989;64:1373-1380.
5. Aubry MC, Wright JL, Myers JL. The pathology of smoking-related lung diseases. Clin Chest Med. 2000;21:11-35, vii.
6. Ryu JH, Myers JL, Capizzi SA, et al. Desquamative interstitial pneumonia and respiratory bronchiolitis-associated interstitial lung disease. Chest. 2005;127:178-184.
7. Tazelaar HD, Wright JL, Churg A. Desquamative interstitial pneumonia. Histopathology. 2011;58:509-516.
8. Nagai S, Hoshina Y, Hayashi M, Ito I. Smoking-related interstitial lung diseases. Curr Opin Pulm Med. 2000;6:415-419.
9. Newman KB, Mason UG 3rd, Schmaling KB. Clinical features of vocal cord dysfunction. Am J Respir Crit Care Med. 1995;152(4, pt 1):1382-1386.
