- Clinical Technology
- Adult Immunization
- Hepatology
- Pediatric Immunization
- Screening
- Psychiatry
- Allergy
- Women's Health
- Cardiology
- Pediatrics
- Dermatology
- Endocrinology
- Pain Management
- Gastroenterology
- Infectious Disease
- Obesity Medicine
- Rheumatology
- Nephrology
- Neurology
- Pulmonology
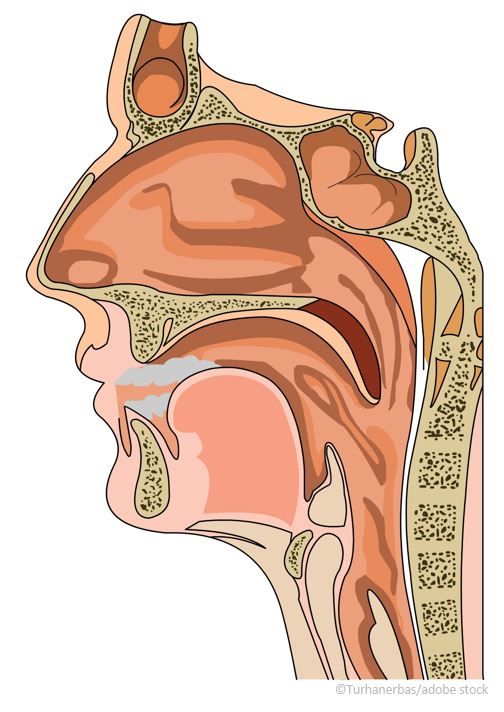
Optinose Xhance approved by FDA as first medical therapy for CRS
Now FDA approved, Optinose’s Xhance is the first nonsurgical option for chronic rhinosinusitis both with and without nasal polyps.
©turhanerbas/stock.adobe.com
UPDATE:
The US Food and Drug Administration (FDA) approved Xhance (fluticasone propionate), a novel treatment-device system that offers the first nonsurgical option for patients with chronic rhinosinusitis (CRS) without nasal polyps, according to a March 15 company news release.4
“The FDA approval of Xhance for the treatment of CRS without nasal polyps is an important milestone,” said Rick Chandra, MD, Professor of Otolaryngology-Head and Neck Surgery at Vanderbilt University, in an Optinose news release. “Until today, we have been forced to use unproven therapies to try and alleviate the symptoms that these patients suffer.”4
CRS is a common chronic condition, impacting approximately 30 million adult patients in the United States. More than two-thirds of the patients suffering from CRS do not have nasal polyps.4
“Although chronic sinusitis is one of the most common diagnoses in outpatient physician visits, and surgery is available, there has never been a prescription medication approved by the FDA as safe and effective to treat the millions of patients without nasal polyps suffering from this debilitating disease,” said Ramy Mahmoud, MD, MPH, the CEO of Optinose, in the news release. "We are thrilled to now be able to offer new hope to these patients and believe Xhance has the potential to become part of the standard of care for the treatment of chronic sinusitis.”4
ORIGINAL ARTICLE:
For individuals with chronic rhinosinusitis (CRS), the US Food and Drug Administration (FDA) approval of Xhance, a novel treatment-device system, scheduled for Saturday, March 16, 2024, would offer the first nonsurgical option for those with the condition both with nasal polyps (CRSwNP) or without.1
The novel drug-device system uses the Exhalation Delivery System (EDS) to deliver fluticasone propionate to regions both high and deep in the nasal cavity where sinuses ventilate and drain, pathways not reached by standard-delivery nasal sprays.2 The FDA approved Xhance for treatment of CRSwNP in adults aged 18 years and older in 2017 based on the Optinose phase 3 ReOpen clinical trial program. The company's supplemental new drug application (sNDA) submitted in May 2023 for the expanded indication for CRS comprised data from the ReOpen1 and ReOpen2 trials that were published in January 2024 in the Journal of Allergy and Clinical Immunology: In Practice.2
Findings from the ReOpen program are the first placebo-controlled evidence to demonstrate the efficacy of any nasal medication in CRS without nasal polyps, including a significant reduction in acute sinusitis exacerbations, ReOpen investigators said in the published study.2 Frequent exacerbations, or flares, impact quality of life (QoL) and importantly also may lead to overuse of antibiotics and oral steroids or even to surgery.
“The ReOpen program evaluated EDS-FLU efficacy on symptoms, QoL, intrasinus inflammation, and acute exacerbations in patients with CRS, particularly those without nasal polyps,” added the investigators. “No medication has previously been shown in replicate, controlled trials to improve symptoms, (quality of life), intrasinus inflammation, and acute exacerbations for this population.”2
ReOpen clinical trial program2
The 2 phase 3 randomized, EDS-placebo–controlled trials enrolled adults with CRS irrespective of polyps (ReOpen1) or exclusively without polyps (ReOpen2) and were conducted at 120 study sites in 13 countries. Patients received EDS-fluticasone (EDS-FLU) 1 or 2 sprays per nostril, or EDS-placebo, twice daily for 24 weeks. The study’s coprimary measures were a patient-reported composite symptom score (congestion, facial pain or pressure, and nasal discharge) through week 4 and an objective measure of average percentage of opacified volume across the ethmoid and maxillary sinuses assessed via CT scan at week 24. (study)
Findings. Among the 332 participants in the ReOpen 1 trial, the composite symptom score least-squares mean (LSM) change for EDS-FLU 1 or 2 sprays/nostril vs EDS-placebo was −1.58 and −1.60 vs −0.62 (P < .001, P < .001). LSM change for sinus opacification for EDS-FLU 1 or 2 sprays/nostril vs EDS-placebo was −5.58 and −6.20 versus −1.60 (P = .045, P = .018) (study).
ReOpen2 enrolled 223 participants. The researchers reported LSM change for the symptom composite score for EDS-FLU 1 or 2 sprays/nostril vs EDS-placebo of −1.54 and −1.74 versus −0.81 (P = .011, P = .001). Findings in ReOpen2 for LSM change for sinus opacification for EDS-FLU 1 or 2 sprays/nostril vs EDS-placebo was −7.00 and −5.14 versus +1.19 (P < .001, P = .009).
In a striking finding, acute disease exacerbations were reduced by 56% to 66% with EDS-FLU versus EDS-placebo (P = .001). There were significant, and similar magnitude, symptom reductions in patients using standard-delivery nasal steroid products just before entering the study (P < .001).
The safety profile and tolerability of Xhance in the ReOpen program were consistent with the product’s currently labeled safety profile.
“Exhalation delivery system with fluticasone is an appropriate first-line treatment for CRS, including when symptoms persist with over-the-counter medications, before escalation to surgery or systemic therapy,” study authors concluded.2
“The burden of chronic sinusitis has been measured and is serious, with a degree of impaired quality of life and functioning comparable to other serious chronic conditions such as chronic heart failure, sciatica, or migraine.” said Ramy Mahmoud, MD, MPH, CEO of Optinose.1 Mahmoud added that chronic sinusitis is one of the top diagnoses in adult outpatient physician visits. “Having a proven effective medication would finally allow doctors to have confidence that they can offer some relief to tens of millions of people suffering from this burdensome inflammatory condition.”1
