- Clinical Technology
- Adult Immunization
- Hepatology
- Pediatric Immunization
- Screening
- Psychiatry
- Allergy
- Women's Health
- Cardiology
- Pediatrics
- Dermatology
- Endocrinology
- Pain Management
- Gastroenterology
- Infectious Disease
- Obesity Medicine
- Rheumatology
- Nephrology
- Neurology
- Pulmonology
Roche, Alnylam Advance Zilebesiran Into Phase 3 CV Outcomes Trial for Resistant Hypertension
Zilebesiran’s twice-yearly dosing and sustained BP reductions could offer a new therapeutic approach for adults with resistant hypertension and high CV risk who are uncontrolled on current SOC regimens.
Roche and Alnylam will initiate a global phase 3 cardiovascular outcomes trial (CVOT) to evaluate zilebesiran, an investigational interfering RNA (RNAi) therapy, for reducing major adverse CV events in adults with uncontrolled hypertension, according to a statement from Roche. The ZENITH phase 3 clinical trial is expected to begin by the end of the year, the company said.1
©Sundry Photos/stock.adobe.com

This next step in the investigation of zilebesiran follows analysis of data from the phase 2 KARDIA program, including KARDIA-1, KARDIA-2, and KARDIA-3, which studied zilebesiran in participants with uncontrolled hypertension and high cardiovascular risk on background treatment with 2 to 4 antihypertensive agents.
KARDIA-3 Outcomes
The codevelopers announced the most recent phase 2 outcomes, from the KARDIA-3 study, in a late-breaking session at the European Society of Cardiology Congress 2025. Researchers reported that a single 300 mg subcutaneous dose of zilebesiran administered every 6 months reduced office systolic blood pressure (SBP) by a placebo-adjusted −5.0 mm Hg at month 3 (P = .043) and −3.9 mm Hg after 6 months of treatment (95% CI: −8.5, 0.7).1
Analysis of response to the 600-mg dose of zilebesiran showed reductions of −3.3 mmHg (P =.183) and −3.6 mm Hg (95% CI: −8.2, 1.0) at months 3 and 6, respectively. The study did not meet the prespecified statistical significance thresholds due to multiplicity testing requirements, according to Roche. However, it did achieve the goal of identifying a patient subgroup likely to receive the greatest benefit from the investigational drug that will be studied in the upcoming phase 3 CVOT.1
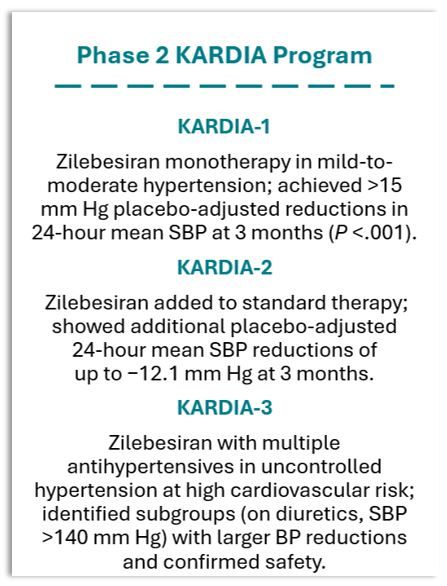
Findings from KARDIA-3 mirrored those from the KARDIA-2 phase 2 study, which demonstrated the benefits of adding zilebesiran to diuretic therapy.2 According to Roche, participants on a diuretic and with baseline SBP greater than 140 mm Hg showed the most significant reductions: −9.2 mmHg (95% CI: −17.3, −1.2) when assessed at month 3 and −8.3 mmHg (95% CI: −16.4, −0.2) at month 6.1
Hypertension affects an estimated 1.28 billion adults aged 30–79 years worldwide, with nearly half unaware they have the disease and only 42% diagnosed and treated, according to the World Health Organization. Remarkably, up to 80% of affected individuals fail to achieve adequate blood pressure control despite available treatments.3
“Zilebesiran has the potential to become a best-in-disease treatment for many patients with uncontrolled hypertension. Its blood pressure-lowering effects and twice-yearly dosing could reduce the risk of serious health complications and death,” Levi Garraway, MD, PhD, Roche’s chief medical officer and head of Global Product Development, said in the statement.1
Zilebesiran was well tolerated when combined with standard-of-care therapies in KARDIA 3; more than 90% of participants were on an ACE inhibitor or angiotensin receptor blocker. Serious adverse events occurred in 3.8% of zilebesiran-treated patients and 4.5% of placebo-treated patients, with no deaths reported in the 6-month double-blind period.
ZENITH: Phase 3 Cardiovascular Outcomes Trial
The ZENITH phase 3 CVOT will enroll approximately 11,000 participants across 30 countries, evaluating zilebesiran 300 mg dosed every 6 months vs placebo in individuals with uncontrolled hypertension despite treatment with at lest 2 antihypertensive drugs, 1 a diuretic, and with either established CV disease or at elevated CV risk, according to Roche. The trial, expected to commence by the end of 2025, will measure the impact of zilebesiran on reduction of risk for CV death, nonfatal myocardial infarction, nonfatal stroke, and heart failure hospitalization or urgent visits.1
Roche and Alnylam are co-developing zilebesiran, which targets angiotensinogen in the renin-angiotensin-aldosterone system using Enhanced Stabilization Chemistry Plus GalNAc conjugate technology, enabling biannual dosing, according to the Roche statement.
References
Roche and Alnylam advance zilebesiran into global phase III cardiovascular outcomes trial for people with uncontrolled hypertension. News release. Roche. August 29, 2025. Accessed September 9, 2025. https://www.roche.com/media/releases/med-cor-2025-08-30
Halsey G. Zilebesiran reduces SBP by up to 14 mm Hg on background SOC in uncontrolled hypertension: KARDIA-2. Patient Care. April 8, 2024. https://www.patientcareonline.com/view/zilebesiran-reduces-sbp-by-up-to-14-mm-hg-on-background-soc
Hypertension. Key facts. World Health Organization. March 16, 2023. Accessed September 9, 2025. https://www.who.int/news-room/fact-sheets/detail/hypertension
