- Clinical Technology
- Adult Immunization
- Hepatology
- Pediatric Immunization
- Screening
- Psychiatry
- Allergy
- Women's Health
- Cardiology
- Pediatrics
- Dermatology
- Endocrinology
- Pain Management
- Gastroenterology
- Infectious Disease
- Obesity Medicine
- Rheumatology
- Nephrology
- Neurology
- Pulmonology
Recurrent Urothelial Bladder Cancer Among HIV-Infected Patients
We report 4 cases of bladder cancer in an ethnically diverse population of about 2500 HIV-infected patients. These patients were younger than the median age at diagnosis of bladder cancer in the United States.
Since the introduction of highly active antiretroviral therapy, the incidence of AIDS-related malignancies, such as Kaposi sarcoma, non-Hodgkin lymphoma, primary brain lymphoma, and cervical cancer, has decreased.1 However, the incidence of non–AIDS-defining cancers, including cancer of the lungs, breast, prostate, skin, colon, and rectum, has increased.2
In HIV-infected persons, urinary bladder cancer is rare; it has been reported in only 13 HIV-infected patients in Europe.3 Gaughan and colleagues4 identified 11 patients with HIV-associated bladder cancer, 7 of whom received highly active antiretroviral therapy; the median age was 58 years, and the male to female ratio was 6:1. Pathology findings at initial diagnosis ranged from low- to high-grade carcinoma. The mean CD4+ cell count was 367/μL (range, 228/μL to 572/μL) for the 7 patients.
We report on 4 cases of urothelial carcinoma of the bladder in ethnically diverse HIV-infected patients at an urban HIV outpatient clinic.
THE CASESCase 1
In 2002, HIV infection was diagnosed in a 65-year-old white woman with a CD4+ cell count of 300/μL and an HIV RNA level of 40,050 copies/mL. She was given zidovudine/lamivudine and nevirapine and had a good response. After 6 months of therapy, laboratory testing revealed a CD4+ cell count of 433/μL and an undetectable viral load.
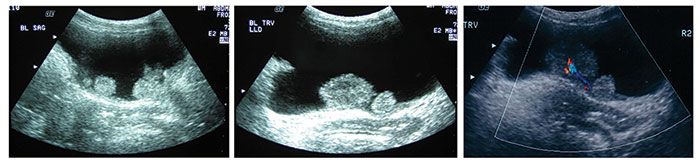
Figure 1. Ultrasonography detected multiple polypoid masses in the bladder of a 65-year-old HIV-infected woman. The largest mass measured 2.4 × 2.1 × 2.9 cm. Pathological examination revealed that a low-grade urothelial carcinoma had invaded the submucosa.
The patient reported recurrent right upper quadrant pain in 2003 but was asymptomatic until 2004, when she presented to the emergency department with persistent abdominal pain and associated difficulty in voiding (the results of urinalysis were not available). Abdominal ultrasonography showed multiple polypoid bladder masses with tumors on the bladder neck and posterior and lateral bladder walls (Figure 1). Two months later, a large (5-cm) mass situated on the trigone and multiple tumors on the bladder neck and posterior and lateral bladder walls were removed through transurethral resection.
Pathological examination revealed that a low-grade, T1 urothelial carcinoma had invaded the submucosa. The patient's CD4+ cell count was 305/μL, and her HIV RNA level was 10,000 copies/mL (the antiretroviral therapy had been discontinued 2 months before laboratory testing). She had been a heavy smoker in the past and smoked 2 or 3 cigarettes a day at the time of diagnosis.
Ten months later, a follow-up cystoscopy showed the presence of 2 medium-sized tumors. The tumors were resected, and mitomycin was instilled intravesically. The biopsy results showed grade 1/3 transitional cell carcinoma without evidence of muscularis invasion. A follow-up cystoscopy 8 months later found no evidence of recurrence. Urinalysis results were negative for blood, and microscopic examination showed 2 to 4 red blood cells per high-power field. The patient remains in remission.
Case 2
A 53-year-old Haitian man was admitted with complaints of fever, chills, cough, weight loss, polyuria, and dysuria. He had received a diagnosis of HIV infection 3 months earlier; he had no history of smoking. He had microscopic hematuria on admission, his CD4+ cell count was 88/μL, and his HIV RNA level was more than 750,000 copies/mL. Blood cultures after 7 days were positive for Mycobacterium tuberculosis. An inguinal node biopsy specimen grew M tuberculosis that was sensitive to all antituberculosis agents.
The patient was given isoniazid, rifabutin, ethambutol, and pyrazinamide for treatment of tuberculosis (TB). Three months after his TB diagnosis, HIV therapy with zidovudine/lamivudine and lopinavir/ritonavir was started. He had a good response to treatment, with an increase in CD4+ cell count to 142/μL and decreased HIV RNA level to 2130 copies/mL.
After antiretroviral therapy was initiated, inguinal lymphadenopathy developed again, associated with fever and weight loss, and an abdominal CT scan showed worsening lymphadenopathy. The patient’s clinical condition was attributed to immune reconstitution inflammatory syndrome (IRIS). His symptoms were self-limited. Microhematuria was noted on urinalysis.
About 1 month after completing a 6-month course of TB treatment, the patient was admitted to the hospital with gross hematuria without other symptoms. A CT scan of the abdomen and pelvis revealed bilateral hydronephrosis and possible bladder wall thickening. His previous lymphadenopathy had decreased. A new biopsy specimen from the inguinal node showed granulomas and acid-fast bacilli. Urine cultures were negative for mycobacteria, but TB therapy was re-initiated. It was thought that IRIS had recurred as a result of TB with genitourinary tract involvement. The patient’s CD4+ cell count was 162/μL, and he had an undetectable viral load.
Two months later, he underwent further workup for persistent hematuria. A CT scan showed a mass in the floor of the bladder with bilateral hydronephrosis. The inguinal lymphadenopathy had decreased further. Cystoscopy revealed a tumor larger than 3 cm that circumferentially filled the bladder neck; transurethral resection was done. A biopsy specimen showed high-grade, T2 urothelial carcinoma with invasion of the muscularis.
The patient was treated with chemotherapy consisting of cisplatin, methotrexate, and vinblastine; however, his disease did not respond to treatment. The team determined that he was not a good candidate for repeated chemotherapy. The patient was discharged to hospice with stage IV bladder cancer and genitourinary TB.
Case 3
A 58-year-old Hispanic man, who had received a diagnosis of HIV infection in 1993, underwent a workup for a self-limited episode of hematuria in 2003. His CD4+ cell count was 116/μL, and his HIV RNA level was 102 copies/mL. His antiretroviral regimen was tenofovir, emtricitabine, and fosamprenavir/ritonavir.
The patient reported a history of smoking 1 or 2 cigarettes per day. He had 1 episode of microscopic hematuria the previous year, and an ultrasonogram had shown a 1.6 × 1.2-cm hypoechoic cyst on the right bladder wall. Hematuria developed again; the results of urine cytology were negative, and CT findings were unremarkable.
Cystoscopy revealed a 3.4-cm papillary tumor at the trigone, which was removed by transurethral resection. Pathological examination revealed a low-grade, T2 papillary urothelial carcinoma with invasion to the detrusor muscle. Mitomycin C was administered postoperatively.
Three months later, a biopsy specimen showed chronic cystitis without malignancy. The results of urine cytology, done at 5 and 8 months, were negative for malignant cells. Ten months later, the patient reported that he was seeing a urologist in the community for recurrence of the cancer. He had a CD4+ cell count of 335/μL and an HIV RNA level of 66 copies/mL. The patient was subsequently lost to follow-up.
Case 4
A 51-year-old African American man presented with frank hematuria in 2004. He had received a diagnosis of HIV infection in 1994, with a nadir CD4+ cell count of 20/μL and an HIV RNA level of more than 750,000 copies/mL. His latest antiretroviral regimen consisted of zidovudine/abacavir/lamivudine, tenofovir, and efavirenz, but these drugs had been withheld before his symptoms developed because of a suspected hypersensitivity reaction to abacavir. His CD4+ cell count was 200/μL, and his HIV RNA level was 100,000 copies/mL.
The patient’s past medical history was significant for latent TB, which was treated with rifabutin and pyrazinamide for 2 months. The patient smoked approximately 10 cigarettes per day.
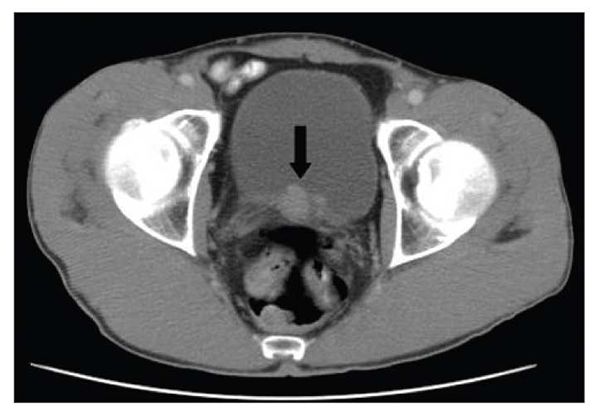
Figure 2. Focal thickening of the right lateral aspect of the bladder wall can be seen in this CT scan. A polypoid filling defect within the bladder was detected on delayed imaging. A 2 × 3-cm diverticulum filled with tumor was extracted by transurethral resection. Pathological examination of the tissue showed a papillary urothelial carcinoma with no muscularis involvement. The patient was a 51-year-old HIV-infected man.
A CT scan of the abdomen and pelvis showed focal thickening of the right lateral wall of the bladder close to the trigone, with a polypoid defect in the inferior aspect (Figure 2). A 2 × 3-cm diverticulum filled with tumor was extracted by transurethral resection. Pathological examination of the tissue showed low-grade, T1 papillary urothelial carcinoma with no muscularis involvement.
Multiple surveillance cystoscopy done at 6-month intervals over 2 years revealed papillary tumors, which were resected. Pathological studies showed low-grade, T1 papillary carcinoma. Cystoscopy done at 1 year showed multiple bladder tumors, which were removed from the bladder neck, left lateral wall, and posterior wall. Pathological examination revealed a low-grade tumor with extension into the lamina propria. Repeated cystoscopy done at 18 months showed multiple bladder tumors, and pathological examination showed low-grade tumor without muscularis involvement. Two years after diagnosis, cystoscopy revealed multiple tumors. Results from transurethral resection ranged from carcinoma in situ to high-grade papillary urothelial cancer. The patient’s CD4+ cell count was 546/μL, and his HIV RNA level was 641 copies/mL.
The patient transferred his urological care to another facility. He reported receiving treatment with intravesical BCG immunotherapy. He also reported he was being treated for metastatic bladder cancer. His response to treatment is unknown.
DISCUSSION
Bladder cancer is among the top-10 diagnosed cancers in the United States, ranking fourth in men and ninth in women; the male to female ratio is 3:1. More than 90% of cases are urothelial bladder cancer, also known as transitional cell carcinoma, which occurs predominantly in patients older than 50 years.5
The most important risk factor for all bladder cancers is smoking, which contributes to approximately 25% to 60% of cases, followed by petrochemical and other industrial exposures. Other well-documented risk factors for bladder cancer are permanent hair dyes, long-term indwelling catheters, and infection with Schistosoma hematobium (associated with squamous metaplasia).6,7
From 2000 to 2005, the median age at diagnosis of bladder cancer in the United States was 73 years; 45.5% of patients were between the ages of 45 and 64 years. Approximately 68,810 persons (51,230 men and 17,580 women)-nearly 3 times more men than women-had cancer of the urinary bladder diagnosed in 2008.5
During 2004-2005, bladder cancer was diagnosed in 4 patients out of a cohort of 1510 HIV-positive patients older than 45 years at our clinic; the age range was 51 to 65 years, with a median age of 57.5 years. Gaughan and colleagues4 reported a median age of 55 years for 11 patients (range, 33 to 67 years), which is significantly younger than the above-mentioned national statistic of 73 years.
Consistent with the typical presentation seen in the non–HIV-infected population, most of our patients were men and had a history of smoking. Overall, our patients had more advanced disease at the time they started antiretroviral therapy. Our cohort had lower mean nadir CD4+ cell counts, 131/μL (range, 20/μL to 300/μL), with 75% having the diagnosis of AIDS. Also, at time of diagnosis of bladder cancer, all patients had lower CD4+ cell counts 192/μL (range, 116/μL to 305/μL).
In the general population, the probability of bladder cancer recurrence ranges from 50% to 90%, depending on the stage, grade, and number of primary tumors. Progression in stage and/or grade occurs in 10% to 50% of cases.8 In our cohort, pathology and stage at initial diagnosis were as follows: The first patient had low-grade (T1N0M0) cancer, the second patient had grade 4 (T2NXM1) cancer, the third patient had low-grade (T2N0M0) tumor with muscularis invasion, and the fourth patient had low-grade (T1N0M0) cancer.
More advanced HIV disease at the time of bladder cancer diagnosis may suggest an association of lower CD4+ cell counts with poorer bladder cancer outcomes. However, no assumptions about these associations can be made given the small cohort sizes. Interestingly, our cluster of patients received the diagnosis during 2004-2005.
Highly active antiretroviral therapy did not appear to alter the course of bladder cancer in our group.
Results from the US Nurses Health Studies cohort showed that postmenopausal status, earlier age at onset of menopause, and age younger than 45 years (compared with age 50 years and older) were associated with a statistically significant increased risk of bladder cancer (relative risk, 1.63; 95% confidence interval, 1.20, 2.23).9
Coinfections With TB
The 2 patients with TB in our group had worse outcomes. In one, cancer was diagnosed after the completion of treatment for TB; the other had a history of treated latent M tuberculosis infection.
CXCR4 chemokine receptors are associated with advanced stages and recurrence of bladder cancer.10 In addition, CXCR4 are co-receptors in HIV disease and facilitate the entry of HIV into target cells. CXCR4-tropic HIV has been associated with faster T-cell depletion and AIDS progression.11,12 CXCR4 expression is increased in active TB. While Rosas-Taraco and colleagues13 showed that TB up-regulates both CCR5 and CXCR4 receptors in monocytes in vitro, Hoshino and associates14 found that macrophages taken from bronchoalveolar lavage specimens from patients with TB preferentially expressed CXCR4. TB may have unregulated this molecule, leading to enhanced pathogenesis of bladder cancer.
CONCLUSIONS
Urinary bladder carcinoma in HIV-seropositive patients has been rare. In our small cohort, 4 of 1510 patients older than 45 years had a significantly lower median age of onset and possibly more aggressive disease, compared with national statistics.
The findings of this case series suggest the need for further study of the increased risk of bladder cancer among the HIV-infected patient population. Studies may be warranted to probe the possible association between HIV-1/M tuberculosis coinfection, CXCR4 expression, and bladder cancer progression.
Survival rates continue to improve for HIV-infected patients, spurring new challenges in research and clinical practice. Non–AIDS-defining cancers are increasing in the HIV-seropositive population, and ongoing efforts will be needed to monitor the incidence of specific cancers, such as bladder cancer, in these patients. Research should be aimed at developing pathways of care and may eventually be required to determine whether urinalysis with microscopic analysis should be included in the routine follow-up care of HIV-positive patients.
Acknowledgments: This work was supported in part by grant 5P30AI073961 from the National Institutes of Health, National Institute for Allergy and Infectious Diseases and the Developmental Center for AIDS Research, University of Miami Miller School of Medicine, Miami (Dr Savita Pahwa).
References:
References1. Nutankalva L, Wutoh AK, McNeil J, Frederick WR, et al. Malignancies in HIV: pre- and post-highly active antiretroviral therapy. J Natl Med Assoc. 2008;100:817-820.
2. Burgi A, Brodine S, Wegner S, et al. Incidence and risk factors for the occurrence of non-AIDS-defining cancers among human immunodeficiency virus-infected individuals. Cancer. 2005;104:1505-1511.
3. Manfredi R, Sabbatani S, Calza L, Chiodo F. Bladder carcinoma and HIV infection during the highly active antiretroviral therapy era: a rare but intriguing association. Two case reports and literature review. Scand J Infect Dis. 2006;38:566-570.
4. Gaughan EM, Dezube BJ, Bower M, et al. HIV-associated bladder cancer: a case series evaluating difficulties in diagnosis and management. BMC Urol. 2009;9:10.
5. Horner MJ, Ries LAG, Krapcho M, et al, eds. SEER Cancer Statistics Review 1975-2006. National Cancer Institute Web site. http://seer.cancer.gov/csr/1975_2006. Accessed October 19, 2009.
6. Dinney CP, McConkey DJ, Millikan RE, et al. Focus on bladder cancer. Cancer Cell. 2004;6:111-116.
7. Johansson SL, Cohen SM. Epidemiology and etiology of bladder cancer. Semin Surg Oncol. 1997;13:291-298.
8. Grossman HB, Soloway M, Messing E, et al. Surveillance for recurrent bladder cancer using a point-of-care proteomic assay. JAMA. 2006;295:299-305.
9. McGrath M, Michaud DS, De Vivo I. Hormonal and reproductive factors and the risk of bladder cancer in women. Am J Epidemiol. 2006;163:236-244.
10. Retz MM, Sidhu SS, Blaveri E, et al. CXCR4 expression reflects tumor progression and regulates motility of bladder cancer cells. Int J Cancer. 2005;114:182-189.
11. Poveda E, Briz V, Quiñones-Mateu M, Soriano V. HIV tropism: diagnostic tools and implications for disease progression and treatment with entry inhibitors. AIDS. 2006;20:1359-1367.
12. Rockstroh JK. Tracking tropism: epidemiology and clinical tools. Module 2 of 4 in: HIV Entry and Tropism: New Strategies for Effective Antiretroviral Therapy. Postgraduate Institute for Medicine, Clinical Care Options. http://www.clinicaloptions.com/HIV/Treatment%20Updates/Tropism/Modules/Rockstroh.aspx. Accessed October 19, 2009.
13. Rosas-Taraco AG, Arce-Mendoza AY, Caballero-OlÃn G, et al. Mycobacterium tuberculosis upregulates coreceptors CCR5 and CXCR4 while HIV modulates CD14 favoring concurrent infection. AIDS Res Hum Retroviruses. 2006;22:45-51.
14. Hoshino Y, Tse DB, Rochford G, et al. Mycobacterium tuberculosis-induced CXCR4 and chemokine expression leads to preferential X4 HIV-1 replication and human macrophages [published correction appears in J Immunol. 2006;177:8874]. J Immunol. 2004;172:6251-6258.
