- Clinical Technology
- Adult Immunization
- Hepatology
- Pediatric Immunization
- Screening
- Psychiatry
- Allergy
- Women's Health
- Cardiology
- Pediatrics
- Dermatology
- Endocrinology
- Pain Management
- Gastroenterology
- Infectious Disease
- Obesity Medicine
- Rheumatology
- Nephrology
- Neurology
- Pulmonology
Pitfalls In Prescribing: Metronidazole Dosing in Patients With Hepatic Dysfunction
Metronidazole, the prototype nitroimidazole, was originally released in 1959 for the treatment of Trichomonas vaginalis infections. It has since been used to treat a variety of infections caused by anaerobic and facultative anaerobic bacteria and protozoa. Here we discuss the need for dosing adjustments in patients with hepatic disease.
Metronidazole, the prototype nitroimidazole, was originally released in 1959 for the treatment of Trichomonas vaginalis infections. It has since been used to treat a variety of infections caused by anaerobic and facultative anaerobic bacteria and protozoa. Here we discuss the need for dosing adjustments in patients with hepatic disease.
METRONIDAZOLE METABOLISM
Metronidazole is extensively metabolized in the liver via the cytochrome P-450 family of enzymes. Five metabolites are produced; the most prevalent one is a hydroxy-metabolite, which has 30% to 65% of the activity of the parent compound.1 The major route of elimination of these metabolites is via the urine (60% to 80% of the dose)2; however, dose adjustment in patients with renal dysfunction is not necessary until the creatinine clearance is less than 10 mL/min.
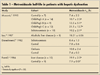
There is a relative lack of literature about the need for dose adjustments in patients with hepatic dysfunction. Table 1 summarizes the results of some of the best studies on this topic. All of these studies show that the half-life and clearance of metronidazole are prolonged in patients with hepatic dysfunction, compared with patients who have normal liver function; however, the volume of distribution of metronidazole is not significantly different from that in controls.3-8
Muscar and associates3 evaluated patients with various Child-Pugh scores and found that the half-life of metronidazole increases and clearance decreases significantly as liver function worsens. The Child-Pugh score is calculated based on levels of bilirubin and albumin, international normalized ratio, and the presence of ascites and encephalopathy; each factor is graded on a 3-point scale. Class A correlates with a score of 5 to 6, class B is 7 to 9, and class C is 10 to 15.

METRONIDAZOLE DOSING
There are no well-defined recommendations for dose adjustments in patients with hepatic dysfunction. However, the increased half-life of metronidazole in patients with hepatic dysfunction may lead to an increased incidence of adverse reactions. The most serious adverse effects are those involving the CNS (Table 2). For example, mental confusion has been reported in a patient with an elevated serum metronidazole concentration.9

Lau and colleagues4 suggest using a 12-hour dosing regimen, rather than the commonly recommended 6-hour regimen, for patients with hepatic dysfunction. Another recommendation is to give 50% of the usual dose every 12 hours to persons with end-stage liver disease.10Table 3 shows the dosages usually given to patients with normal liver function as well as possible adjustments for those with hepatic dysfunction.
References:
REFERENCES:
1. Jensen JC, Gugler R. Single- and multiple-dose metronidazole kinetics. Clin Pharmacol Ther. 1983;34:481-487.
2. Flagyl [package insert]. Chicago: GD Searle LLC; 2004.
3. Muscará MN, Pedrazzoli J Jr, Miranda EL, et al. Plasma hydroxy-metronidazole/metronidazole ratio in patients with liver disease and in healthy volunteers. Br J Clin Pharmacol. 1995;40:477-480.
4. Lau AH, Evans R, Chang CW, Seligsohn R. Pharmacokinetics of metronidazole in patients with alcoholic liver disease. Antimicrob Agents Chemother. 1987;31:1662-1664.
5. Daneshmend TK, Homeida M, Kaye CM, et al. Disposition of oral metronidazole in hepatic cirrhosis and in hepatosplenic schistosomiasis. Gut. 1982;23:807-813.
6. Farrell G, Baird-Lambert J, Cvejic M, Buchanan N. Disposition and metabolism of metronidazole in patients with liver failure. Hepatology. 1984;4:722-726.
7. Ljungberg B, Nilsson-Ehle I, Ursing B. Metronidazole: pharmacokinetic observations in severely ill patients. J Antimicrob Chemother. 1984;14:275-283.
8. Plaisance KI, Quintiliani R, Nightingale CH. The pharmacokinetics of metronidazole and its metabolites in critically ill patients. J Antimicrob Chemother. 1988;21:195-200.
9. Schentag JJ, Ziemniak JA, Greco JM, et al. Mental confusion in a patient treated with metronidazole-a concentration-related effect? Pharmacotherapy. 1982;2:384-387.
10. Salvatore M, Meyers B. Metronidazole. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and BennettÃs Principles and Practice of Infectious Diseases. 6th ed. Philadelphia: Elsevier; 2005:388-396.
