- Clinical Technology
- Adult Immunization
- Hepatology
- Pediatric Immunization
- Screening
- Psychiatry
- Allergy
- Women's Health
- Cardiology
- Pediatrics
- Dermatology
- Endocrinology
- Pain Management
- Gastroenterology
- Infectious Disease
- Obesity Medicine
- Rheumatology
- Nephrology
- Neurology
- Pulmonology
Novel GLP-1 RA for Obesity Shows Promise in Early Phase 1 Trial
Once-daily oral TERNS-601 was associated with a significant mean 5% weight reduction after 28 days and had a favorable tolerability profile.
©Postmodern Studio/stock.adobe.com

A novel once-daily oral glucagon-like peptide 1 receptor agonist (GLP-1 RA) being investigated by Terns Pharmaceuticals, demonstrated a significant 5.5% mean loss of bodyweight over 28 days in healthy adults with obesity or overweight, according to the company. Topline findings from the phase 1 dose-finding study showed that 67% of participants lost 5% or more of their baseline bodyweight at the top dose of TERN-601. The new incretin mimetic was well tolerated across the 3 doses evaluated, according to a Terns press release, with no reported treatment-related dose interruptions, reductions, or discontinuations despite the rapid dose titration used in the trial.
“We are delighted to demonstrate potent GLP-1R agonism with TERN-601 as its distinct drug properties allowed for sustained target coverage with once-daily dosing and the evaluation of doses up to 740 mg, while being tolerable,” Emil Kuriakose, chief medical officer of Terns, said in the press statement. “Importantly, we believe we have successfully identified an optimal range of clinically active, well tolerated doses to take forward in Phase 2 clinical trials, with no new dose range exploration anticipated.”
During the randomized, double-blind, placebo controlled single and multiple ascending dose (SAD, MAD) trial, researchers assessed the safety, tolerability, pharmacokinetics, and pharmacodynamics of TERN-601 compared with placebo. The 36 adults with overweight or obesity in the 28-day MAD study were randomly assigned to receive 240 mg, 500 mg or 740 mg TERN-601 or placebo once daily (9 participants in each group).
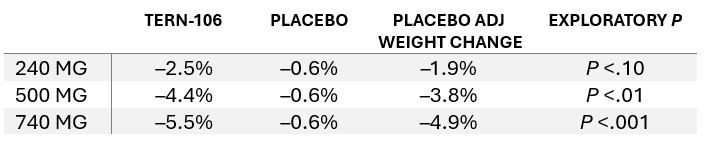
According to Terns, the majority (<95%) of adverse events were mild, including gastrointestinal events that were consistent with the GLP-1 RA class. Researchers reported no clinically meaningful changes in liver enzymes, vital signs, or ECG results. The overall favorable tolerability of TERN-601 in this study despite “aggressive titration to high doses,” augurs well for future research with slower titration schedules, said Tern.
Specific properties of the novel drug could offer benefits over current GLP-1 RA formulations, the company suggested. “Its low solubility and high gut permeability may result in prolonged absorption allowing for sustained target coverage and a flat [pharmacokinetics] curve, while high drug levels in the gut wall may lead to robust GLP-1 [receptor] activation in the gut, triggering satiety centers in the brain,” according to the press release. “Additionally, TERN-601 has a low free fraction in circulation which, combined with the flat [pharmacokinetics] curve, may be allowing TERN-601 to be well-tolerated when administered at high doses.” The selection of TERN-601 during the company’s drug-discovery process puts a “potent GLP-1 [receptor] agonist based toward cAMP generation” into the rapidly growing field of investigational antiobesity medications.
“These compelling results underscore TERN-601’s potential to be a class-leading GLP-1R agonist based on its composite profile of initial indications of efficacy, tolerability, and manufacturing scalability,” Amy Burroughs, chief executive officer of Terns, said in the press release. “With operational preparations well underway, we look forward to swiftly advancing this promising product candidate into Phase 2 clinical development in 2025.”
Source: Terns Pharmaceuticals announces positive phase 1 clinical trial results with Tern-601 once-daily oral GLP-1R agonist for the treatment of obesity. News release. Terns Pharmaceuticals, Inc. September 9, 2024. Accessed September 9, 2024. https://ir.ternspharma.com/news-releases/news-release-details/terns-pharmaceuticals-announces-positive-phase-1-clinical-trial
Obesity Linked to Faster Alzheimer Disease Progression in Longitudinal Blood Biomarker Analysis
December 2nd 2025Biomarker trajectories over 5 years in study participants with AD show steeper rises in pTau217, NfL, and amyloid burden among those with obesity, highlighting risk factor relevance.
