- Clinical Technology
- Adult Immunization
- Hepatology
- Pediatric Immunization
- Screening
- Psychiatry
- Allergy
- Women's Health
- Cardiology
- Pediatrics
- Dermatology
- Endocrinology
- Pain Management
- Gastroenterology
- Infectious Disease
- Obesity Medicine
- Rheumatology
- Nephrology
- Neurology
- Pulmonology
Longitudinal Blood Biomarkers Show Promise for Monitoring Alzheimer's Disease Progression in Subjective Cognitive Decline
Blood-based monitoring could enable earlier intervention as approximately 20% of initially negative participants transitioned to positive biomarker status.
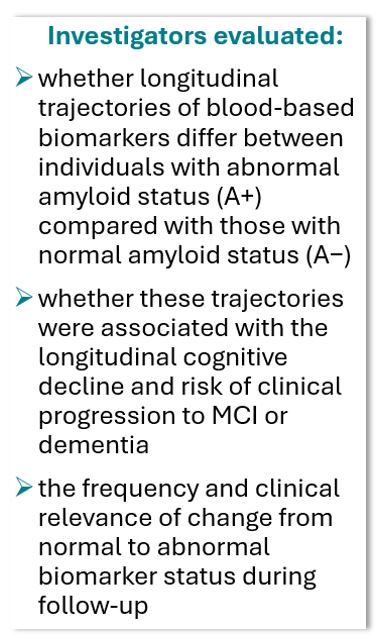
A prospective cohort study of 298 individuals with subjective cognitive decline (SCD) has found that longitudinal increases in plasma phosphorylated tau 217 (pTau217) and glial fibrillary acidic protein (GFAP) are strongly associated with cognitive decline and clinical progression to mild cognitive impairment (MCI) or dementia.
©SpeedKingz/stock.adobe.com

The findings suggest these blood-based biomarkers could serve as practical tools for early identification and monitoring of Alzheimer disease (AD) progression in at-risk individuals, according to the research team from the Netherlands. Although there are published data from longitudinal studies in preclincal stages, the focus has been largrely on cognitively unimpaired indviduals and they have "rarely investigated associations between biomarker changes and clinical progression,"2,3 they wrote.
The current study, conducted at the Alzheimer Center Amsterdam and published in JAMA Network Open, followed participants for a mean of 4.8 years (maximum 15.6 years) with biennial biomarker collection and annual cognitive assessment. At baseline, 80 of the study participants (26.8%) were amyloid-positive (A+) and 218 (73.2%) were amyloid-negative (A−). During follow-up, 33 participants (11.1%) progressed to MCI or dementia.
Biomarker Trajectories and Cognitive Decline
For pTau217, GFAP, and neurofilament light (NfL), baseline levels were higher in the A+ group compared with the A− group (estimates [SE] amyloid β, 1.11 [0.11], 0.69 [0.13], and 0.36 [0.10], respectively; P <.001 for all). These biomarkers also showed steeper increases over time in the A+ group (estimates [SE] time × amyloid status β, 0.07 [0.02], P <.001; 0.07 [0.02], P <.001; and 0.05 [0.02], P =.005, respectively).
In contrast, while individuals with A+ had a lower amyloid-β42/40 ratio (Aβ42/40) at baseline (estimate [SE] amyloid status β = −0.39 [0.05]; P <.001), there were no differences in rate of change between groups, reflecting what the authors described as "an early plateau in the disease process and limiting its monitoring utility."
Longitudinal increases in pTau217 and GFAP were associated with cognitive decline across all domains (β time × biomarker slope = −0.02 to −0.04). Decreases in Aβ42/40 were associated with decline only in global cognition (β = 0.03 [0.01], P =.04) and language (β = 0.04 [0.02], P = .03), while increases in NfL were associated with decline in global cognition (β = −0.02 [0.01], P =.004), language (β = −0.03 [0.01], P =.007), and executive functioning (β = −0.03 [0.01], P =.02).
Prognostic Value
According to Cox proportional hazards regression models:
- Steeper pTau217 slope was associated with progression from SCD to MCI or dementia (hazard ratio [HR], 3.6; 95% CI, 1.8-7.4 per 0.05 SD increase per year; C index, 0.89; 95% CI, 0.84-0.93)
- Steeper GFAP slope was also associated with progression (HR, 1.5 [95% CI, 1.0-2.2]; C index, 0.81 [95% CI, 0.73-0.88])
- Steeper NfL slope, too, was associated with progression (HR, 2.6 [95% CI, 1.3-5.2]; C index, 0.77 [95% CI, 0.69-0.85])
- Aβ42/40 slope was not associated with progression
When the researchers combined baseline and slope values using elastic net regularization, the optimal model included baseline Aβ42/40 and both baseline and slope values for pTau217 and GFAP, yielding a C index of 0.90 (95% CI, 0.87-0.94).
Negative to Positive Conversion Over Time
Approximately 1 in 5 participants who were biomarker negative at baseline converted to positive during follow-up: Aβ42/40, 25 (11.2%); pTau217, 50 (24.0%); GFAP, 66 (36.7%); and NfL, 54 (32.9%). Those who developed positive pTau217, GFAP, or NfL status showed steeper cognitive declines compared with those who remained biomarker negative. "Unlike stable negative participants, who improved in cognition (likely due to learning effects), those transitioning to pTau217 positivity showed no improvements, placing their trajectory between the stable negative and stable positive groups," authors wrote.
Clinical Implications
The study addresses an important gap in understanding biomarker trajectories in preclinical AD in particular, authors stressed. Abnormal accumulation of amyloid β and tau proteins begins decades before clinical symptoms are evident, and while having abnormal AD-related blood-based biomarkers during preclinical stages is associated with future cognitive decline, less is known about their longitudinal evolution.
"Individuals with SCD from the memory clinic represent a key population for early detection and intervention," the team pointed out, "because they actively present with cognitive issues while carrying an increased risk of progression compared with cognitively unimpaired individuals." They emphasized that "with the advent of disease-modifying treatments, these patients constitute an easily targetable group within SCD trial-ready cohorts and are expected to play an important role in preclinical prevention trials."
The investigators reiterate the potential for blood-based biomarkers in cogntive research, ie, their non-invasive, cost-effective, and easily repeatable nature, which makes them promising alternatives to cerebrospinal fluid and positron emission tomography assessments for longitudinal monitoring. While current guidelines do not recommend biomarker testing in preclinical stages,4,5 the findings strengthen the evidence that blood-based biomarkers are reliable indicators of cognitive decline and clinical progression on a group level. The authors caution that "before such individual-level prediction can be realized, additional longitudinal studies in SCD cohorts are needed to replicate and harmonize findings across research settings."
References
Trieu C, van Harten AC, van Leeuwenstijn MSSA, et al. Lontitudinal blood-based biomarkers and clinical progression in subjective cognitive decline. JAMA Netw Open. 2025 2025;8;(12):e2545862. doi:10.1001/jamanetworkopen.2025.45862
Ashton NJ, Janelidze S, Mattsson-Carlgren N, et al. Differential roles of Aβ42/40, p-tau231 and p-tau217 for Alzheimer’s trial selection and disease monitoring. Nat Med. 2022;28(12):2555-2562. doi:10.1038/s41591-022-02074-w
Chatterjee P, Pedrini S, Ashton NJ, et al. Diagnostic and prognostic plasma biomarkers for preclinical Alzheimer’s disease. Alzheimers Dement. 2022;18(6):1141-1154. doi:10.1002/alz.12447
Jack CR Jr, Andrews JS, Beach TG, et al. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimers Dement. 2024;20(8):5143-5169. doi:10.1002/alz.13859
Dubois B, Villain N, Schneider L, et al. Alzheimer disease as a clinical-biological construct: an international working group recommendation. JAMA Neurol. 2024;81(12):1304-1311. doi:10.1001/jamaneurol.2024.3770
