- Clinical Technology
- Adult Immunization
- Hepatology
- Pediatric Immunization
- Screening
- Psychiatry
- Allergy
- Women's Health
- Cardiology
- Pediatrics
- Dermatology
- Endocrinology
- Pain Management
- Gastroenterology
- Infectious Disease
- Obesity Medicine
- Rheumatology
- Nephrology
- Neurology
- Pulmonology
FDA Accepts NDA for Delgocitinib Cream, Developed Specifically to Treat Chronic Hand Eczema
Delgocitinib specifically inhibits activation of JAK-STAT signaling in chronic hand eczema and would be an option for people unable to use topical corticosteroids.
LEO Pharma announced yesterday that the US FDA has accepted the company’s New Drug Application (NDA) for delgocitinib cream 20 mg/g (2%), an investigational topical treatment for chronic hand eczema (CHE).1
The novel pan-Janus kinase (JAK) inhibitor specifically inhibits the activation of JAK-STAT signaling, a primary trigger in the pathogenesis of this skin disease. If approved, delgocitinib would be the first therapy approved in the US with a specific indication for adults with moderate-to-severe CHE who have responded inadequately to, or are not able to use, topical corticosteroids. There are currently no FDA-approved treatments for CHE.1
Chronic hand eczema affects nearly 10% of the US adult population and has far-reaching physical and psychological sequelae.
(©Ольга Тернавская/stock.adobe.com)
(©Ольга Тернавская/stock.adobe.com)
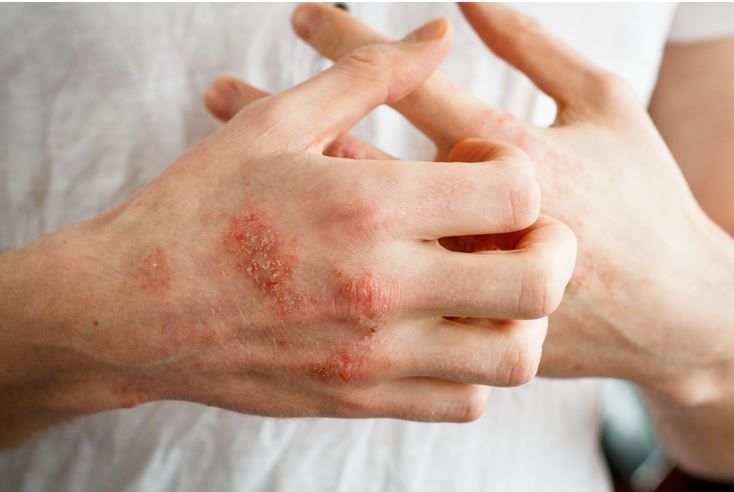
“Dermatologists regularly see the true extent to which CHE impacts patients’ lives through unique physical and psychological challenges,” Christopher Bunick, MD, PhD, associate professor of dermatology at Yale Medical School, said in a company statement.1 "Many patients need more options beyond traditional topical corticosteroid use, so I welcome today’s news, which brings the prospect of a potential new treatment option one step closer for those across the US living with this debilitating condition.”1
CHE is a heterogeneous, inflammatory skin condition with a pathophysiology characterized by barrier dysfunction and alterations in the skin microbiome, the company explained.1 The disease, both pruritic and painful, affects approximately 10% of the US population2 and is known to have a significant impact on individual’s social and psychological wellbeing and to affect the ability to work in gainful employment. The manifestation of CHE can include lichenification, hyperkeratosis, scaling, and fissures on the hands and wrists, according to LEO Pharma.1
The FDA based its acceptance of the regulatory submission on review of findings from LEO Pharma’s phase 3 DELTA 1 and DELTA 2 clinical trials. The studies evaluated the safety and efficacy of delgocitinib 2% applied twice daily compared with a cream vehicle in adults with moderate-to-severe CHE over a 16-week treatment period.3
Both trials met the primary and all secondary endpoints. In DELTA 1, 20% of study participants treated with delgocitinib met the study’s primary endpoint, Investigator’s Global Assessment for Chronic Hand Eczema treatment success (IGA-CHE TS) score of 0 or 1, compared with 10% of those using cream vehicle. In DELTA 2, 29% of those treated with the active study drug achieved IGA-CHE TS vs 7% of the placebo-treated group.3
Participants who completed 16 weeks of treatment with either delgocitinib or placebo in either trial were eligible to continue in the 36-week open label DELTA 3 extension trial.
“With today’s announcement, we reinforce our commitment to addressing the high unmet need of US patients living with CHE, a condition that can significantly impact quality of life, including an impact [on] mental wellbeing,” Brian Hilberdink, EVP and president of Region North America at LEO Pharma, said in the LEO Pharma statement. The company anticipates the US regulatory review to conclude in the second half of 2025.1
