- Clinical Technology
- Adult Immunization
- Hepatology
- Pediatric Immunization
- Screening
- Psychiatry
- Allergy
- Women's Health
- Cardiology
- Pediatrics
- Dermatology
- Endocrinology
- Pain Management
- Gastroenterology
- Infectious Disease
- Obesity Medicine
- Rheumatology
- Nephrology
- Neurology
- Pulmonology
FDA Accepts Dupilumab sBLA for Review for Treatment of Chronic Spontaneous Urticaria
The target action date for the FDA decision is April 18, 2025, according to Sanofi and Regeneron.
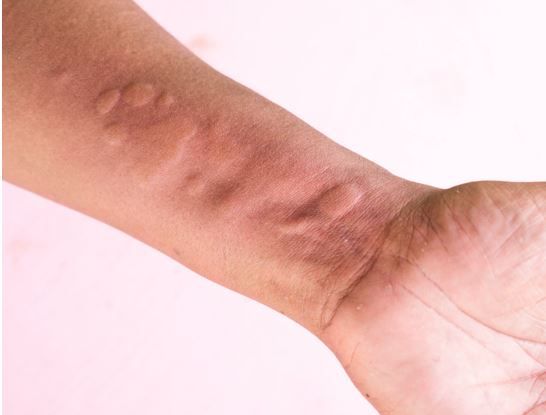
Sanofi and Regeneron Pharmaceuticals announced today that the US FDA has accepted for review the resubmission of the supplemental biologics license application (sBLA) for dupilumab (Dupixent) in treating uncontrolled chronic spontaneous urticaria (CSU). The IL-4 and IL-13 inhibitor is intended for adults and children aged 12 years and older who have not previously reached controlled disease status with H1 antihistamine treatment.
The resubmission is supported by positive results from the phase 3 LIBERTY-CUPID clinical trial program evaluating dupilumab for CSU consisting of study A, study B, and study C. According to the press release, study A and C were conducted in patients with CSU who were uncontrolled on standard-of-care antihistamines and study B examined patients with CSU who were uncontrolled on standard-of-care antihistamines and refractory or intolerant to omalizumab.
Specifically, the sBLA adds results from study C, which met its primary and secondary endpoints in biologic-naïve patients, confirming results previously observed in study A. Results from study C showed dupilumab significantly reduced itch and urticaria activity, according to the press release.
Safety results in all 3 LIBERTY-CUPID studies were generally consistent with the existing safety profile of dupilumab’s approved indications. Adverse events more commonly found with dupilumab compared to placebo (≥5%) were injection site reactions and COVID-19 diagnoses, according to Sanofi and Regeneron.
The target action date for the FDA decision is April 18, 2025. If approved, this would be the first targeted therapy for CSU in more than 10 years.
Reference: Dupixent® (dupilumab) sBLA accepted for FDA review for the treatment of chronic spontaneous urticaria (CSU). News release. Regeneron Pharmaceuticals. November 15, 2024. Accessed November 15, 2024. https://newsroom.regeneron.com/news-releases/news-release-details/dupixentr-dupilumab-sbla-accepted-fda-review-treatment-chronic
