- Clinical Technology
- Adult Immunization
- Hepatology
- Pediatric Immunization
- Screening
- Psychiatry
- Allergy
- Women's Health
- Cardiology
- Pediatrics
- Dermatology
- Endocrinology
- Pain Management
- Gastroenterology
- Infectious Disease
- Obesity Medicine
- Rheumatology
- Nephrology
- Neurology
- Pulmonology
Dupilumab Becomes First Biologic Approved in the US as Targeted Therapy for Adults with COPD
Dupilumab represents the first new treatment approach for uncontrolled COPD in more than a decade and reduced disease exacerbations by 34% in clinical trials.
Sanofi and Regeneron Pharmaceuticals announced today that the US FDA has approved the biologic dupilumab (Dupixent) as add-on maintenance therapy for adults with inadequately controlled chronic obstructive pulmonary disease (COPD) and evidence of an eosinophilic phenotype. With the approval, dupilumab becomes the first biologic agent approved in the US to treat the chronic lung disease and the first new treatment approach for COPD in more than a decade.1
©Waldenmarus/stock.adobe.com

Clinical trials leading to today's approval demonstrated that adults with COPD had significant reductions in annualized disease exacerbations and experienced improvements in both lung function and health-related quality of life, according to the Regeneron announcement.1
“In my more than 20 years of practice, there have been limited advancements for patients struggling with the debilitating effects of uncontrolled COPD, and too many patients experience a vicious cycle of exacerbations that can result in loss of lung function and greatly diminish their quality of life,” coprincipal investigator Surya Bhatt, MD, MSPH, professor, division of pulmonary, allergy, and critical care medicine, at the University of Alabama at Birmingham, said in an earlier statement.2 “In [phase 3 trial] NOTUS, dupilumab reduced exacerbations by a magnitude never seen before with an investigational biologic in a phase 3 COPD clinical study." This is a first of its kind medical advancement for the COPD community, Bhatt said.2
The fully human monoclonal antibody inhibits both interleukin-4 and -13 (IL-4, IL-13), a mechanism of action demonstrated across the dupilumab development program to decrease T2 inflammation in patients with atopic or allergic diseases. Identifying the central role of IL-4 and IL 13 in the type 2 inflammatory cascade led to the pivotal studies of inhibiting both as a means of reducing COPD exacerbations in individuals with a blood eosinophil level of 300 or more cells per mL.1
Pivotal Phase 3 Trials
The FDA decision is based on pivotal replicate phase 3 clinical trials, BOREAS (2023) and NOTUS (2024), which analyzed the safety of dupilumab specifically in adults who were current or former smokers whose COPD symptoms were uncontrolled and who had evidence of T2 inflammation. All study participants in the NOTUS and BOREAS trials were already receiving maximal standard of care inhaled therapy, with the majority prescribed triple therapy.1
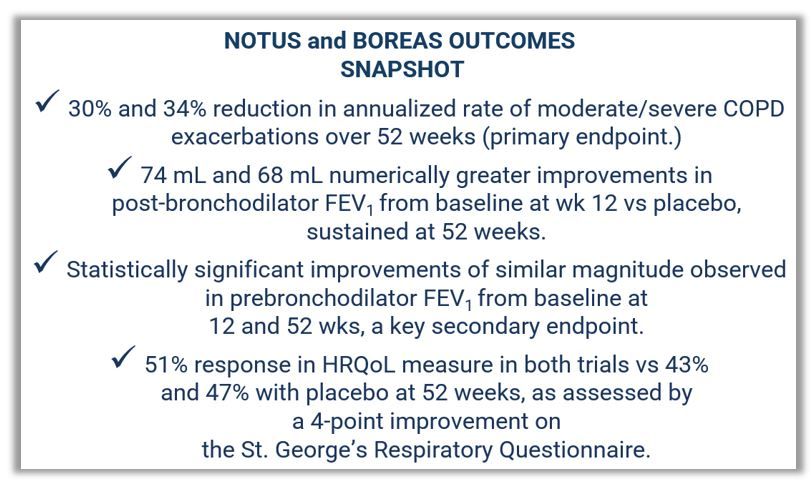
The NOTUS trial, published in the New England Journal of Medicine in May,3 met its primary endpoint “with overwhelming efficacy,” the companies reported, showing that dupilumab significantly reduced annualized moderate or severe COPD exacerbations by 34% compared with placebo.3 The replicate phase 3 BOREAS trial returned equivalent results, with disease exacerbations reduced by 30%. Sanofi and Regeneron reported that improvements in lung function were “rapid and significant,” observed in some dupilumab-treated patients as early as 12 weeks into treatment. The gains were sustained at 52 weeks of follow-up, the companies stated.1
The NOTUS trial participants (n=935) were aged 40 to 85 years and in BOREAS (n=939) were aged 40 to 80 years. All participants were current or former smokers, had evidence of an eosinophilic phenotype, and were receiving maximal standard-of-care inhaled therapy.
During the 52-week treatment period in both trials, participants were randomly assigned to receive dupilumab or placebo every 2 two weeks added to a maximal standard-of-care inhaled triple therapy of inhaled corticosteroids, long-acting ß-agonists, and long-acting muscarinic antagonists.1
Key findings from NOTUS3
- 34% reduction in moderate or severe acute COPD exacerbations over 52 weeks (P <.001), the primary endpoint.
- More than 2 times greater improvement in lung function from baseline at week 12 for dupilumab (139 mL in FEV1) vs placebo (57 mL) (P <.001), with the benefit vs placebo sustained at week 52 (115 mL in FEV1 for dupilumab vs 54 mL for placebo, P = .0182), both of which were key secondary endpoints.
Key findings from BOREAS1
- 30% reduction in moderate or severe acute COPD exacerbations over 52 weeks (P = .0005), the primary endpoint.
- Improved lung function from baseline by 160 mL in FEV1 at 12 weeks compared to 77 mL for placebo (P <.001), with the benefit versus placebo sustained through week 52 (P = .003).
In both trials investigators reported a 51% response in HRQoL compared with 43% and 47% with placebo at 52 weeks based on a 4-point improvement on the internationally utilized St George's Respiratory Questionnaire.1
There were no new safety signals reported for dupilumab across the 2 trials, for a safety profile consistent with the medication in its other approved indications, the company said.1
“Dupixent has already shown it can revolutionize the treatment paradigm of many diseases driven in part by type 2 inflammation with high unmet medical needs, with one million patients being treated globally across all currently approved indications,” Paul Hudson, Sanofi chief executive officer said in the statement.1 “With today’s approval, Dupixent once again paves the way and becomes the first and only approved add-on biologic medicine for inadequately controlled COPD, giving patients living with this devastating disease the chance to look forward to the potential of improved breathing and a life with fewer exacerbations.”1
Dupilumab was reviewed by the FDA under Priority Review and was also approved in China today and by the European Commission for the same indication.1