- Clinical Technology
- Adult Immunization
- Hepatology
- Pediatric Immunization
- Screening
- Psychiatry
- Allergy
- Women's Health
- Cardiology
- Pediatrics
- Dermatology
- Endocrinology
- Pain Management
- Gastroenterology
- Infectious Disease
- Obesity Medicine
- Rheumatology
- Nephrology
- Neurology
- Pulmonology
Diagnostic Nucleic Acid Testing for Invasive Fungal Infections
Over the past 2 decades, there has been an alarming increase in opportunistic fungal infections with an associated rise in morbidity and mortality. This trend has been attributed to the growing number of patients who are immunocompromised because of bone marrow or solid organ transplant, immunosuppressive drugs, AIDS, and hematological malignancies. Advances in trauma and critical care medicine that lead to longer survival of more patients with immunocompromising conditions also play a role.
Over the past 2 decades, there has been an alarming increase in opportunistic fungal infections with an associated rise in morbidity and mortality. This trend has been attributed to the growing number of patients who are immunocompromised because of bone marrow or solid organ transplant, immunosuppressive drugs, AIDS, and hematological malignancies. Advances in trauma and critical care medicine that lead to longer survival of more patients with immunocompromising conditions also play a role.
Historically, the most common opportunistic mycotic infections have been those associated with Candida albicans, Aspergillus fumigatus, and Cryptococcus neoformans. Anumber of fungal pathogens, including non-fumigatus Aspergillus species and other septate moulds, as well as members of the Zygomycetes class are emerging as important causes of fungal disease.1,2 These infections are frequently fatal. Early recognition of these pathogens is critical to initiating prompt, appropriate therapy.
Because all antifungal drugs, including the newer agents, have gaps in coverage, most pathogens should be identified at the species level when devising a therapeutic strategy. However, conventional reliance on culture and histopathology for diagnosis of invasive fungal infections is time-consuming and frequently insensitive. For this reason, nucleic acid-based assays are gaining attention as potentially sensitive, accurate, and rapid tests for the diagnosis of fungal infections.
Overview of molecular diagnostics
Methods that are currently used to diagnose fungal infections include direct observation (smears, histopathology); culture of clinical specimens; and antigen/antibody assays for detecting the cell wall components galactomannan (GM) and ?-glucan. More recently, polymerase chain reaction (PCR) amplification and its variants (including multiplex PCR, nested PCR, and real-time PCR) have been used to detect fungal pathogens, such as Candida and Aspergillus species, by amplifying genomic sequences unique to each organism.3-6
Multiplex PCR provides increased sensitivity over standard PCR by using multiple primer pairs per reaction to amplify more than 1 target sequence.4 Nested PCR, in which the original target sequence is amplified and then used as a template for additional amplifications with a second set of primers, is more specific than conventional PCR. Nested PCR has been used clinically for the detection of Candida species and Histoplasma capsulatum.7-10 Real-time PCR couples the assay with an amplification product detection system (typically a fluorescent label) and has been used to detect and quantify DNA from several fungal pathogens, including Aspergillus species, Candida species, and C neoformans.4,11-15
Fungal nuclear, mitochondrial, and ribosomal genes, as well as RNA sequences, have been used as templates in PCR and similar assays.16 The sensitivity of these assays is enhanced when the target sequence has multiple copies within the genome.14,16 Ribosomal targets possess both sequences, which are highly conserved among the fungi and species-specific variable internal transcribed spacer regions. Recent studies have focused on 5.8S, 18S, and 28S ribosomal RNAand DNAgenes for the detection of Candida and Aspergillus species.7,8,10,11,13,15-17
Once target sequences are amplified by PCR, the amplicons can be further characterized by other molecu-lar biology techniques, including restriction fragment length polymorphism analysis, nucleic acid sequencing, Southern and Northern blot analysis, electrophoretic karyotyping, and DNA microarray genotyping.4,5,18
Clinical application of molecular diagnostics
Although PCR assays can be used to detect any fungi, their clinical application has mostly been applied to the detection of Candida and Aspergillus species.13,15,19,20-26 PCR assays for Candida are very sensitive and can detect DNAfrom as few as 10 organisms/mL of blood. Similarly, PCR assays for Aspergillus can detect DNA from 10 to 100 conidia/mL of blood.24
Pryce and colleagues13 suggested that real-time PCR testing, which can detect DNA quantity over time, might be useful for monitoring response to antifungal therapy. Klingspor and Jalal15 also found real-time PCR assays to be both sensitive and specific for the detection of Candida and Aspergillus species in clinical specimens. In their study, clinical samples (blood, sputum, tissue, cerebrospinal fluid, bronchoalveolar lavage fluid, pleural fluid, ascites, bile, and urine) from transplant recipients with suspected invasive fungal infections were assayed by PCR. Of 1650 specimens assayed, 114 (6.9%) were PCR-positive for either Candida species (n = 86) or Aspergillus species (n = 28), whereas 62 (3.8%) were culturepositive for either Candida species (n = 57) or Aspergillus species (n = 5). Of the PCR samples positive for Candida, 72% were identifiable to species.
Ahmad and colleagues7 found semi-nested PCR to be 99% specific and more sensitive than culture in diagnosing candidemia. White and colleagues17 found that PCR testing, when compared with latex agglutination and enzyme- linked immunosorbent assay, detected Candida infection earlier, was more sensitive, and was comparably specific.
It has been suggested that environmental contaminants might cause false-positive PCR results when used in the diagnosis of fungal infections.17 This has been substantiated in a study by Ljungman and colleagues21 in which blood samples from patients with leukemia were assayed weekly by PCR for Cytomegalovirus and fungi. Real-time PCR results were positive in 9 samples taken from 8 of 35 patients (3 samples positive for Aspergillus and 5 samples positive for Candida, with 1 sample being positive for both).21 However, only 3 of the 4 samples in which Aspergillus species were detected corresponded with suspicion for Aspergillus infection based on the presence of pulmonary infiltrates on a chest CT scan.21
In the same study, of 3 cases of proven fungemia attributed to Candida species, in only 1 case was the blood PCR-positive for Candida.21 Six samples from 5 patients were PCR-positive for Candida species.21 Two samples came from 1 patient who had bacteremia, 1 sample came from an asymptomatic patient, 1 sample each came from 2 patients with fever of unknown origin, and 1 sample came from a patient with candidemia. Hence, Candida was never recovered in culture of specimens taken from 4 of the 5 patients whose blood was PCR-positive for Candida.21 The PCR test results may represent true positives, although, even in the absence of growth in culture, Candida species are not always recovered from the blood of patients with candidiasis.
Another technique useful for the diagnosis of fungal infections is fluorescence in situ hybridization (FISH). This assay uses fluorescein-labeled peptide nucleic acid (PNA) probes specific for the ribosomal RNA sequences of C albicans.27 The FDAapproved 1 of these assays, the C albicans PNA FISH (AdvanDx, Inc, Woburn, Mass), in 2004 for use in rapidly (ie, within 2.5 hours) differentiating albicans from non-albicans Candida species isolated from blood. In one study, the reported sensitivity and specificity of this technique was 99% and 100%, respectively. 27 The same company also manufactures a dual color C albicans/Candida glabrata FISH assay for simultaneous identification of both organisms from blood culture. The sensitivity and specificity of this dual assay are similar to those of the C albicans PNA FISH.28
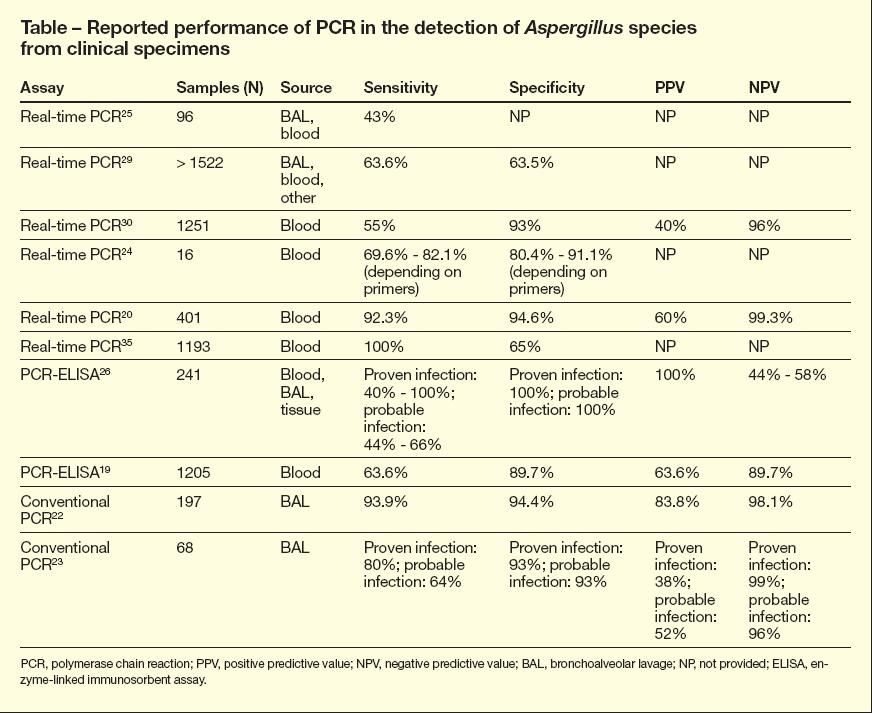
The value of PCR in the diagnosis of invasive aspergillosis has been evaluated in several studies (Table). The sensitivity, specificity, negative predictive value, and positive predictive value vary widely between studies depending on the pretest probability of infection and type of PCR used (eg, real-time vs nested).22-25 The low positive predictive value of PCR assays when bronchoalveolar lavage specimens are used (range, 38% to 83.5%) likely reflects the difficulty in distinguishing airway colonization from infection.22-25
Table
The sensitivity of PCR assays in detecting Aspergillus in serum varies from 40% to 92.3%, with improved sensitivity on serial testing.19,20,26,29 The low sensitivity of the assay described in some studies (especially in early infection) might be attributed to transient fungemia, low-level fungemia (ie, below the detection limits of the assay), and a short half-life of fungal DNA (because of rapid degradation or clearance). PCR testing fares better in detecting Aspergillus invasion of tissue, such as lung tissue, with a reported sensitivity of 100% in one study.26
Comparisons of the GM assay and real-time PCR assay in detecting Aspergillus infections have shown conflicting results. Buchheidt and colleagues29 reported that the nested PCR assay is more sensitive than the GM assay, whereas both Kawazu and colleagues30 and Costa and colleagues31 reported the GM assay to be superior. If valid, the findings of the latter 2 studies might reflect greater shedding of fungal antigen relative to the presence of Aspergillus nucleic acid in the blood of patients with fungemia.
Merits and limitations of molecular diagnostics
When compared with culture and histopathology for the diagnosis of invasive fungal infections, PCR coupled with various hybridization techniques offers the potential of enhanced sensitivity, specificity, and relative rapidity. Moreover, real-time PCR such as the LightCycler PCR detection system (Roche Applied Science, Mannheim, Germany) confers the advantage of quantifying fungal DNA and potentially might be used to monitor disease progression as well as response to therapy.25,26 PCR testing also permits identification of individual species and strains as well as amplification of specific sequences for further study (eg, nucleic acid sequencing) and manipulation (eg, cloning).
However, there are drawbacks to using PCR testing in the diagnosis of infection. The techniques for extracting and amplifying DNA are not currently standardized, and the reactions are expensive and vulnerable to false positive results due to contamination. Most important, positive PCR results may not distinguish between contamination, colonization, or true infection, nor between DNA extracted from dead versus viable organisms.32 Lack of recovery of live organisms also removes the option of performing antifungal susceptibility or retrospective virulence or strain testing. Nevertheless, a great potential value of PCR derives from its negative predictive value.
Conclusions
Studies show PCR assays to be both sensitive and specific in the diagnosis of infections caused by fungi such as
Aspergillus
and
Candida
; sensitivity is typically greater with tissue than with blood. The diagnostic value of PCR testing may be further enhanced in the appropriate clinical setting or when the test is done in conjunction with other tests, such as culture and the GM assay. When done serially, quantitative PCR testing might be useful for monitoring disease progression or response to therapy, and potentially it could be used to differentiate colonization from infection. In addition, PCR testing has shown promise in the diagnosis of infections caused by other fungi, such as
C neoformans, H c
apsulatum,
and
Pneumocystis jiroveci
.
9,33,34
However, the sensitivity of these assays predispose them to false-positive results, and true-positive results may not distinguish between contamination, colonization, and infection. Further clinical studies are needed before PCR testing alone can be advocated for the diagnosis of fungal infection.
References:
- Pfaller MA, Pappas PG, Wingard JR. Invasive fungal pathogens: current epidemiological trends. Clin Infect Dis. 2006;43(suppl 1):S3-S14.
- Nucci M, Anaissie E. Emerging fungi. Infect Dis Clin North Am. 2006;20:563 579.
- O’Shaughnessy EM, Shea YM, Witebsky FG. Laboratory diagnosis of invasive mycoses. Infect Dis Clin North Am. 2003;17:135-158.
- Iwen PC. Molecular detection and typing of fungal pathogens. Clin Lab Med. 2003;23:781-799.
- 5. Alexander BD, Pfaller MA. Contemporary tools for the diagnosis and management of invasive mycoses. Clin Infect Dis. 2006;43(suppl 1):S15-S27.
- Yeo SF, Wong B. Current status of nonculture methods for diagnosis of invasive fungal infections. Clin Microbiol Rev. 2002;15:465-484.
- Ahmad S, Khan Z, Mustafa AS, Khan ZU. Seminested PCR for diagnosis of candidemia: comparison with culture, antigen detection, and biochemical methods for species identification. J Clin Microbiol. 2002;40:2483-2489.
- Ahmad S, Mustafa AS, Khan Z, et al. PCR-enzyme immunoassay of rDNA in the diagnosis of candidemia and comparison with amplicon detection by agarose gel electrophoresis. Int J Med Microbiol. 2004;294:45-51.
- Bialek R, Feucht A, Aepinus C, et al. Evaluation of two nested PCR assays for detection of Histoplasma capsulatum DNAin human tissue. J Clin Microbiol. 2002;40:1644-1647.
- Nazzal D, Yasin S, Abu-Elteen K. Arapid PCR-based method for identification of four important Candida species. New Microbiol. 2005;28:245-250.
- Hsu M, Chen K, Lo H, et al. Species identification of medically important fungi by use of real-time LightCycler PCR. J Med Microbiol. 2003;52(pt 52): 1071-1076.
- O’Sullivan CE, Kasai M, Francesconi A, et al. Development and validation of a quantitative real-time PCR assay using fluorescence resonance energy transfer technology for detection of Aspergillus fumigatus in experimental invasive pulmonary aspergillosis. J Clin Microbiol. 2003;41:5676-5682.
- Pryce TM, Kay ID, Palladino S, Heath CH. Real-time automated polymerase chain reaction (PCR) to detect Candida albicans and Aspergillus fumigatusDNA in whole blood from high-risk patients. Diagn Microbiol Infect Dis. 2003;47: 487-496.
- Bu R, Sathiapalan RK, Ibrahim MM, et al. Monochrome LightCycler PCR assay for detection and quantification of five common species of Candida and Aspergillus. J Med Microbiol. 2005;54(pt 3):243-248.
- Klingspor L, Jalal S. Molecular detection and identification of Candida and Aspergillus spp. from clinical samples using real-time PCR. Clin Microbiol Infect. 2006;12:745-753.
- Yeo SF, Wong B. Current status of nonculture methods for diagnosis of invasive fungal infections. Clin Microbiol Rev. 2002;15:465-484.
- White PL, Archer AE, Barnes RA. Comparison of non-culture-based methods for detection of systemic fungal infections, with an emphasis on invasive Candida infections. J Clin Microbiol. 2005;43:2181-2187.
- Gil-Lamaignere C, Roilides E, Hacker J, Muller FM. Molecular typing for fungi-a critical review of the possibilities and limitations of current and future methods. Clin Microbiol Infect. 2003;9:172-185.
- 19. Florent M, Katsahian S, Vekhoff A, et al. Prospective evaluation of a polymerase chain reaction-ELISA targeted to Aspergillus fumigatus and Aspergillus flavus for the early diagnosis of invasive aspergillosis in patients with hematological malignancies. J Infect Dis. 2006;193:741-747.
- White PL, Linton CJ, Perry MD, et al. The evolution and evaluation of a whole blood polymerase chain reaction assay for the detection of invasive aspergillosis in hematology patients in a routine clinical setting. Clin Infect Dis. 2006;42:479-486.
- Ljungman P, von Döbeln L, Ringholm L, et al. The value of CMV and fungal PCR for monitoring of acute leukaemia and autologous stem cell transplant patients. Scand J Infect Dis. 2005;37:121-127.
- Buchheidt D, Baust C, Skladny H, et al. Clinical evaluation of a polymerase chain reaction assay to detect Aspergillus species in bronchoalveolar lavage samples of neutropenic patients. Br J Haematol. 2002;116:803-811.
- Raad I, Hanna H, Huaringa A, et al. Diagnosis of invasive pulmonary aspergillosis using polymerase chain reaction-based detection of Aspergillus in BAL. Chest. 2002;121:1171-1176.
- White PL, Barton R, Guiver M, et al. Aconsensus on fungal polymerase chain reaction diagnosis? A United Kingdom-Ireland evaluation of polymerase chain reaction methods for detection of systemic fungal infections. J Mol Diagn. 2006;8:376-384.
- Spiess B, Buchheidt D, Baust C, et al. Development of a LightCycler PCR assay for detection and quantification of Aspergillus fumigatus DNAin clinical samples from neutropenic patients. J Clin Microbiol. 2003;41:1811-1818.
- 26. Lass-Flörl C, Gunsilius E, Gastl G, et al. Diagnosing invasive aspergillosis during antifungal therapy by PCR analysis of blood samples. J Clin Microbiol. 2004;42:4154-4157.
- Forrest GN, Mankes K, Jabra-Rizk MA, et al. Peptide nucleic acid fluorescence in situdhybridization-based identification of Candida albicans and its impact on mortality and antifungal therapy costs. J Clin Microbiol. 2006;44:3381- 3383.
- Wu FP, Della-Latta R, Addison B, et al. Dual color PNA FISH assay for simultaneous identification of Candida albicans and Candida glabrata directly from positive blood culture bottles. In: Proceedings from Infectious Disease Society of America 2006 General Meeting; October 12-15, 2006; Toronto. Abstract 06-LB2053.
- Buchheidt D, Hummel M, Schleiermacher D, et al. Prospective clinical evaluation of a LightCycler-mediated polymerase chain reaction assay, a nested- PCR assay and a galactomannan enzyme-linked immunosorbent assay for detection of invasive aspergillosis in neutropenic cancer patients and haematological stem cell transplant recipients. Br J Haematol. 2004;125:196- 202.
- Kawazu M, Kanda Y, Nannya Y, et al. Prospective comparison of the diagnostic potential of real-time PCR, double-sandwich enzyme-linked immunosorbent assay for galactomannan, and a (1â>3)-beta-D-glucan test in weekly screening for invasive aspergillosis in patients with hematological disorders. J Clin Microbiol. 2004;42:2733-2741.
- Costa C, Costa J, Desterke C, et al. Real-time PCR coupled with automated DNA extraction and detection of galactomannan antigen in serum by enzyme- linked immunosorbent assay for diagnosis of invasive aspergillosis. J Clin Microbiol. 2002;40:2224-2227.
- Bretagne S. Molecular diagnostics in clinical parasitology and mycology: limits of the current polymerase chain reaction (PCR) assays and interest of the real-time PCR assays. Clin Microbiol Infect. 2003;9:505-511.
- Bialek R, Weiss M, Bekure-Nemariam K, et al. Detection of Cryptococcus neoformans DNA in tissue samples by nested and real-time PCR assays. Clin Diagn Lab Immunol. 2002;9:461-469.
- Larsen H, Masur H, Kovacs J, et al. Development and evaluation of a quantitative, touch-down, real-time PCR assay for diagnosing Pneumocystis carinii pneumonia. J Clin Microbiol. 2002;40:490-494.
- Hebart H, Löffler J, Meisner C, et al. Early detection of Aspergillus infection after allogeneic stem cell transplantation by polymerase chain reaction screening. J Infect Dis. 2000;181:1713-1719.
