- Clinical Technology
- Adult Immunization
- Hepatology
- Pediatric Immunization
- Screening
- Psychiatry
- Allergy
- Women's Health
- Cardiology
- Pediatrics
- Dermatology
- Endocrinology
- Pain Management
- Gastroenterology
- Infectious Disease
- Obesity Medicine
- Rheumatology
- Nephrology
- Neurology
- Pulmonology
Atumelnant for Congenital Adrenal Hyperplasia Agent Granted FDA Orphan Drug Designation
The FDA's ODD award is based on the positive findings from the phase 2 TouCAHn trial, which also support Crinetics' robust phase 3 pivotal clinical trial program for the novel agent.
The FDA granted Orphan Drug Designation (ODD) to Crinetics Pharmaceuticals’ atumelnant, an investigational once-daily oral adrenocorticotropic hormone (ACTH) receptor antagonist for the treatment of classic congenital adrenal hyperplasia (CAH). The company announced the award on August 21, 2025.1
©pikovit/stock.adobe.com
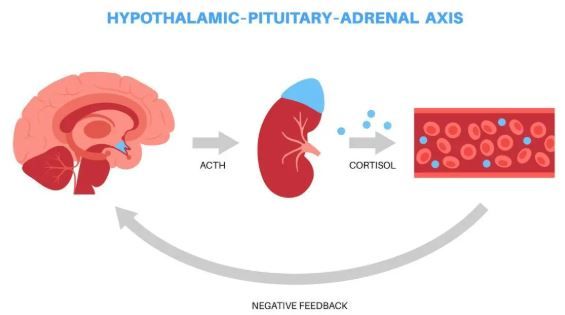
The agency's decision was based on topline results reported in January from the phase 2 TouCAHn trial of atumelnant in adults with classic CAH.2 Across doses, the drug produced rapid and sustained reductions of key biomarkers, including up to an 80% mean reduction in androstenedione (A4). Investigators also documented improvements in clinical signs and symptoms such as resumption of menses and reduction of adrenal size.2
Before the end of 2025, Crinetics plans to begin random assignment of participants in 2 late-stage trials of atumelnant: the CALM-CAH phase 3 study in adults and the BALANCE-CAH Phase 2/3 pediatric study.
“Receiving Orphan Drug Designation from the FDA underscores the significant unmet need faced by people living with CAH,” Dana Pizzuti, MD, chief medical and development officer of Crinetics, said in a statement. “Through atumelnant’s innovative mechanism of action, we have developed an ambitious and uncompromising endpoint for our phase 3 trial, which can demonstrate the ability to restore normal levels of adrenal androgens and reduce glucocorticoid supplementation to physiologic levels. We will also document other changes in clinical disease markers and symptoms that improve quality of life for patients.”
First-in-class. Atumelnant acts selectively at the melanocortin type 2 receptor (MC2R) on the adrenal gland. In preclinical models, it demonstrated strong binding affinity for MC2R and suppression of adrenally derived glucocorticoids and androgens under ACTH control, according to the Crinetics statement. In a 12-week phase 2 study, atumelnant produced statistically significant reductions in disease-related biomarkers, including androstenedione (A4) and 17-hydroxyprogesterone, in a diverse patient population.
Successful Phase 2 Trial
The open-label, 12-week phase 2 TouCAHn study enrolled 28 adults aged 18 years or older to age 75 with classic CAH on stable glucocorticoid replacement. Participants were assigned to one of 3 atumelnant dose cohorts (40 mg, 80 mg, or 120 mg once daily). The primary endpoints were changes in morning serum (A4) levels and the incidence of treatment-emergent adverse events. Secondary endpoints included changes in morning serum 17-hydroxyprogesterone (17-OHP) levels.2
The topline safety findings from TouCAHn showed atumelnant was generally well-tolerated, with no reports of treatment-related severe or serious adverse events (SAEs) across dose levels or disease severity, according to Crinetics.2 The most common AEs were mild to moderate headaches (25%) and fatigue (18%). No participants required dose reductions or discontinued treatment due to AEs. Across all dose groups, no clinically significant trends were observed in laboratory parameters, vital signs, electrocardiograms, or physical examinations.2
“There has been a long-standing interest in using a potent, selective antagonist of the ACTH receptor for the treatment of CAH and other diseases of ACTH excess, leading to the design of atumelnant by Crinetics scientists,” Alan Krasner, MD, chief endocrinologist of Crinetics, said in an earlier statement on the TouCAHn results.3 “This Phase 2 study demonstrated that atumelnant was well tolerated and resulted in a reduction of adrenal androgen levels so rapid and robust that it allowed patients to realize meaningful improvements in long-term, pre-existing medical challenges, even within the short 12-week treatment period of this study.”3
CAH is caused by genetic mutations that impair cortisol synthesis, leading to persistently elevated ACTH levels and overproduction of androgens such as androstenedione. Excess androgens may cause reduced fertility in both sexes, excessive facial hair and acne in women, and testicular adrenal rest tumors in men. Current management requires lifelong glucocorticoid supplementation, often at supraphysiologic levels, which can lead to complications including obesity, diabetes, cardiovascular disease, and osteoporosis.
References
Crinetics receives FDA orphan drug designation for atumelnant in the treatment of congenita adrenal hyperplasia (CAH). News release. Crinetics Pharmaceuticals. August 21, 2025. Accessed August 21, 2025. https://crinetics.com/crinetics-receives-fda-orphan-drug-designation-for-atumelnant-in-the-treatment-of-congenital-adrenal-hyperplasia-cah/
Halsey G. Atumelnant demonstrates rapid and sustained biomarker reduction in phase 2 trial in adults with congenital adrenal hyperplasia. Patient Care. January 10, 2025. https://www.patientcareonline.com/view/atumelnant-demonstrates-rapid-and-sustained-biomarker-reduction-in-phase-2-trial-in-adults-with-congenital-adrenal-hyperplasia
Crinetics announces positive topline results from phase 2 trial of atumelnant in congenital adrenal hyperplasia (CAH). News release. Crinetics Pharmaceuticals, Inc. January 10, 2025. Accessed August 21, 2025. https://crinetics.com/crinetics-announces-positive-topline-results-from-phase-2-trial-of-atumelnant-in-congenital-adrenal-hyperplasia-cah/
