- Clinical Technology
- Adult Immunization
- Hepatology
- Pediatric Immunization
- Screening
- Psychiatry
- Allergy
- Women's Health
- Cardiology
- Pediatrics
- Dermatology
- Endocrinology
- Pain Management
- Gastroenterology
- Infectious Disease
- Obesity Medicine
- Rheumatology
- Nephrology
- Neurology
- Pulmonology
Arcutis Begins Study of Roflumilast Cream 0.05% in Infants with Atopic Dermatitis
Arcutis Biotherapeutics announced today the enrollment of the first patient in the phase 2 INTEGUMENT-INFANT trial.
©Юля Шевцова/AdobeStock
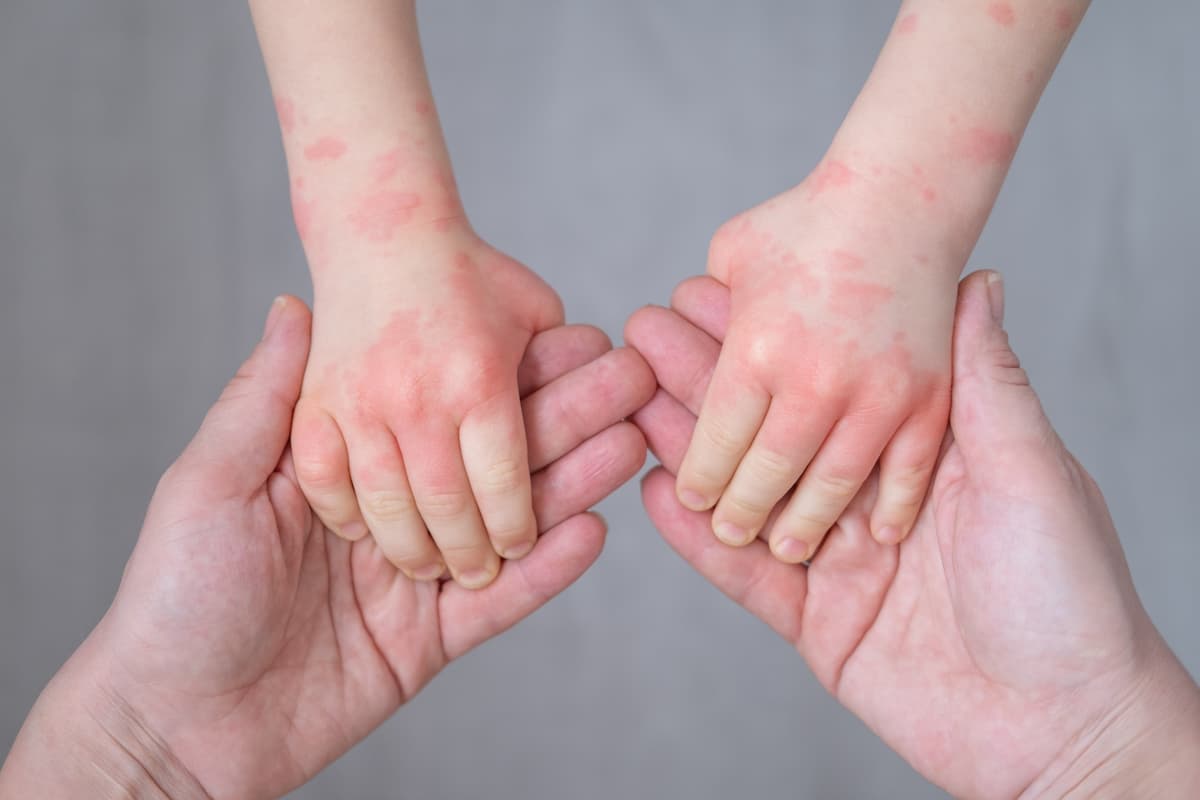
Arcutis Biotherapeutics announced the enrollment of the first patient in the phase 2 INTEGUMENT-INFANT trial, a four-week open-label study evaluating the safety and tolerability of investigational roflumilast (Zoryve®) cream 0.05% in infants aged 3 months to less than 2 years with atopic dermatitis (AD).1
The study will include approximately 35 infants with mild to moderate AD involving at least 3% body surface area (BSA). The cream will be applied once daily for four weeks. The primary objective is to assess safety and tolerability in this age group, who currently have limited therapeutic options.1
“Despite the high prevalence, early onset, and serious impact of AD, there are very few topical or systemic therapies approved for infants, making new clinical research for tolerable and effective treatments that can be used over a lifetime and can reduce or replace steroids, critically important for this age group. Enrolling the first child in this study is a meaningful step forward and builds on our mission to address unmet needs in pediatric dermatology,” Patrick Burnett, MD, PhD, FAAD, chief medical officer at Arcutis, said in a press release today.1
The trial builds on results from the ARQ-151-105 (MUSE) study, which evaluated roflumilast cream 0.05% in the same age group. Roflumilast is a highly potent and selective phosphodiesterase-4 (PDE4) inhibitor designed for topical use. PDE4 is a well-established target in dermatology, regulating pro-inflammatory and anti-inflammatory mediators.1
AD affects approximately 9.6 million children in the US. Up to 60% of affected children develop symptoms within the first year of life, often presenting as red, itchy, scaly patches on the cheeks, scalp, or body. The early onset and chronic nature of AD frequently lead to disrupted sleep, increased risk of infection, and psychological stress for both children and caregivers.
Roflumilast cream 0.15% is currently FDA approved for the treatment of mild-to-moderate AD in individuals aged 6 years and older.2 The 0.05% formulation is under FDA review for use in children aged 2 to 5 years, with a Prescription Drug User Fee Act (PDUFA) target action date of October 13, 2025.3
If successful, the INTEGUMENT-INFANT study would expand the age range for which roflumilast is available, offering a nonsteroidal, targeted topical treatment option for infants—an underserved and vulnerable population in dermatology.1
References:
1. Arcutis Enrolls First Child in INTEGUMENT-INFANT Evaluating ZORYVE® (roflumilast) Cream 0.05% in Infants with Atopic Dermatitis. News release. Arcutis Biotherapeutics. June 10, 2025. Accessed June 10, 2025. https://investors.arcutis.com/news-releases/news-release-details/arcutis-enrolls-first-child-integument-infant-evaluating-zoryver
2. Halsey G. Roflumilast Cream 0.15% Approved for Treatment of Atopic Dermatitis in People Aged 6 Years and Older. Patient Care Online. July 11, 2023. https://www.patientcareonline.com/view/roflumilast-cream-0-15-approved-for-treatment-of-atopic-dermatitis-in-people-aged-6-years-and-older
3. Halsey G. sNDA for Roflumilast Cream 0.05% for the Treatment of AD in Children Aged 2 to 5 Years Gets FDA Nod. Patient Care Online. March 3, 2025. https://www.patientcareonline.com/view/snda-for-roflumilast-cream-0-05-for-the-treatment-of-ad-in-children-aged-2-to-5-years-gets-fda-nod
Atopic Dermatitis: The Pipeline and Clinical Approaches That Could Transform the Standard of Care
September 24th 2025Patient Care tapped the rich trove of research and expert perspectives from the Revolutionizing Atopic Dermatitis 2025 conference to create a snapshot of the AD care of the future.
