- Clinical Technology
- Adult Immunization
- Hepatology
- Pediatric Immunization
- Screening
- Psychiatry
- Allergy
- Women's Health
- Cardiology
- Pediatrics
- Dermatology
- Endocrinology
- Pain Management
- Gastroenterology
- Infectious Disease
- Obesity Medicine
- Rheumatology
- Nephrology
- Neurology
- Pulmonology
Baxdrostat Granted FDA Priority Review for Hard-to-Control Hypertension, Could Be First-In-Class Therapy
The Priority Review decision is based on phase 3 results showing consistent SBP reductions across uncontrolled and treatment-resistant hypertension subgroups.
The US Food and Drug Administration (FDA) has accepted AstraZeneca’s New Drug Application (NDA) for baxdrostat under Priority Review for the treatment of adults with hard-to-control hypertension, including treatment-resistant cases.
The company expects the Prescription Drug User Fee Act (PDUFA) date in the second quarter of 2026. If approved, baxdrostat could become the first aldosterone synthase inhibitor authorized for clinical use.1
©Ihsanfrr/stock.adobe.com Generated with AI
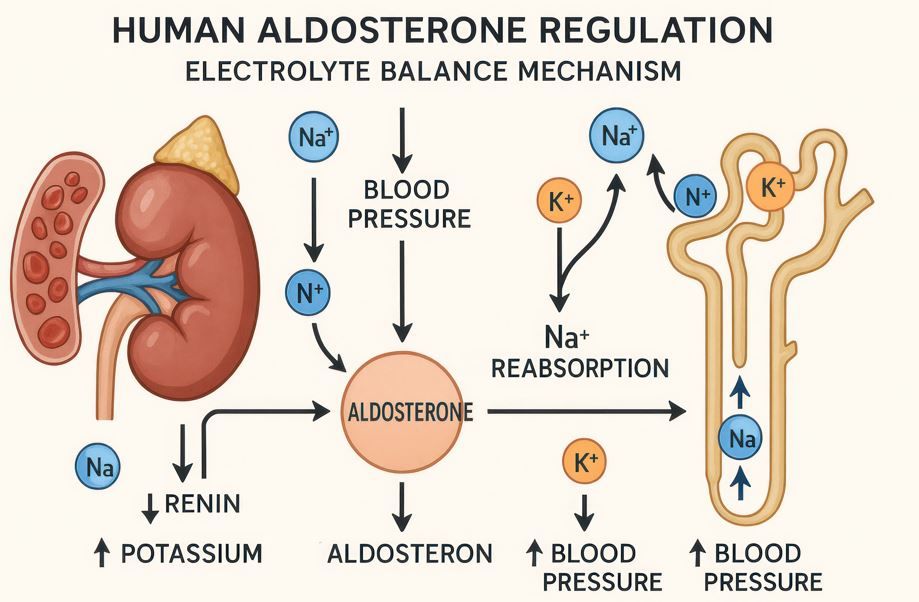
The baxdrostat NDA submission is supported by results from the BaxHTN phase III trial (NCT06034743)2,3 which demonstrated statistically significant and clinically meaningful reductions in systolic blood pressure (SBP) when added to standard-of-care antihypertensive therapy. In the double-blind, placebo-controlled study, 796 patients with uncontrolled or treatment-resistant hypertension were randomized 1:1:1 to receive baxdrostat 2 mg, 1 mg, or placebo once daily for 12 weeks.2,3
At week 12, patients receiving baxdrostat 2 mg experienced a mean seated SBP reduction of 15.7 mmHg (95% CI, -17.6 to -13.7) from baseline and a placebo-adjusted reduction of 9.8 mmHg (95% CI, -12.6 to -7.0; P <.001). The 1 mg dose produced a 14.5 mmHg (95% CI, -16.5 to -12.5) baseline reduction and 8.7 mmHg (95% CI, -11.5 to -5.8; P <.001) placebo-adjusted reduction. These results were consistent across patients with uncontrolled hypertension and those with treatment-resistant hypertension, which investigators defined as persistently elevated blood pressure despite therapy with 3 or more antihypertensives, including a diuretic.3
Baxdrostat was generally well tolerated, and most adverse events were mild. Investigators observed no unanticipated safety findings, and its profile aligned with the drug’s mechanism of action as a selective aldosterone synthase inhibitor.3
“This Priority Review demonstrates our commitment to advancing baxdrostat as a potential first- and best-in-class aldosterone synthase inhibitor for the millions of people living with hard-to-control hypertension as quickly as possible,” Sharon Barr, EVP, BioPharmaceuticals R&D, said in a statement. “The substantial reduction in systolic blood pressure seen in the BaxHTN trial underscores baxdrostat’s novel mechanism of action and its potential to bring innovation to a disease area that has seen limited progress in over two decades.”1
Baxdrostat works by specifically inhibiting aldosterone synthase, the enzyme responsible for production of aldosterone, which contributes to a hypertensive state and increases cardiovascular and renal risk.4,5 Clinical trials have shown that baxdrostat significantly lowers aldosterone levels without affecting cortisol,6 and ongoing studies are evaluating the drug in more than 20,000 patients globally as monotherapy, in primary aldosteronism, and in combination with dapagliflozin for chronic kidney disease and prevention of heart failure, according to AstraZeneca.1
Hypertension affects an estimated 1.4 billion people worldwide,7 and in the US, about 50% of patients on multiple therapies fail to achieve adequate blood pressure control.7 Persistent elevations in SBP, even modest increases, substantially raise the risk of cardiovascular events. Observational data show that a 9.5 mmHg increase in SBP associates with a 30% higher risk of all-cause mortality and a 41% higher risk of cardiovascular death.8 Increased night-time blood pressure and morning blood pressure surges also correlate with higher cardiovascular risk.8
The BaxHTN trial included a randomized withdrawal phase (weeks 24–32) to assess the persistence of efficacy, and a long-term safety assessment at week 52 compared with standard care. Secondary endpoints evaluated diastolic blood pressure changes, the proportion of participants achieving SBP under 130 mmHg
Baxdrostat could address a significant unmet need in patients whose hypertension remains uncontrolled despite multiple medications, offering a targeted mechanism to reduce aldosterone-driven blood pressure elevation.
References
- AstraZeneca. Baxdrostat new drug application accepted under FDA Priority Review in the US for patients with hard-to-control hypertension. News release. December 2, 2025. https://www.astrazeneca-us.com/content/az-us/media/press-releases/2025/Baxdrostat-New-Drug-Application-accepted-under-FDA-Priority-Review-in-the-US-for-patients-with-hard-to-control-hypertension.html. Accessed December 2, 2025.
- ClinicalTrials.gov. A study to investigate the efficacy and safety of baxdrostat in participants with uncontrolled hypertension on two or more medications including participants with resistant hypertension (BaxHTN). ClinicalTrials.gov Identifier: NCT06034743. https://clinicaltrials.gov/study/NCT06034743. Accessed November 2025.
- Flack JM, et al. Efficacy and safety of baxdrostat in uncontrolled and resistant hypertension. N Engl J Med. 2025;393:1363-1374.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension. 2018;71(6):1269-1324.
- Inoue K, Nakanishi R, Takeuchi H, et al. Serum aldosterone concentration, blood pressure, and coronary artery calcium: the multi-ethnic study of atherosclerosis. Hypertension. 2020;76(1):113-120.
- Freeman MW, Cheng S, Desai A, et al. Results from a phase 1, randomized, double-blind, multiple ascending dose study characterizing the pharmacokinetics and demonstrating the safety and selectivity of the aldosterone synthase inhibitor baxdrostat in healthy volunteers. Hypertens Res. 2023;46(1):108-118.
- Carey RM, Calhoun DA, Bakris GL, et al. Prevalence of apparent treatment-resistant hypertension in the United States. Hypertension. 2019;73(2):424-431.
- Niiranen TJ, Mäki J, Puukka P, Karanko H, Jula AM. Office, home, and ambulatory blood pressures as predictors of cardiovascular risk. Hypertension. 2014;64(2):281-286. doi:10.1161/HYPERTENSIONAHA.114.03292.
